Covid19_DB
Covid19_DB is a curated listing of interventional clinical trials in Covid-19/2019-nCoV produced by the Anticancer Fund. Source data comes from ClinicalTrials.gov, the European Clinical Trials Register and WHO ICTRP. Given our expertise in the area of drug repurposing and the urgent needs presented by the Covid-19 pandemic we feel it is socially responsible that we apply our skills to this very different disease area. The intention is to provide researchers, clinicians and regulators with an easily filtered database of interventional trials. As the number and range of trials has continued to increase we have decided to list only those interventional trials which are testing a drug or blood/cell-based product - this includes drug repurposing, shelved compounds, new molecular entities and immunological interventions using plasma, vaccines, stem cells and so on. By making its database open-access and keeping it up-to-date, the Anticancer Fund wants to help to encourage collaboration and alert researchers to the wide range of hypotheses being explored around the world. Ideally this should result in more collaborations between clinicians investigating similar compounds.
As of 20/08/20 this database is no longer being actively updated. The initial urgency of the situation has passed and there are now a number of other actively managed databases providing similar data and functionality to this one. Good examples are the The COVID19 Clinical Trials Explorer and the Living mapping of Covid-19 studies project. Our preprint, outlining our rationale, methodology and results, has been updated to reflect the data as of the end date of our project.
Cite as: Pan Pantziarka, Liese Vandeborne, Lydie Meheus, Gauthier Bouche (2020).Covid19db -- An online database of trials of medicinal products to prevent or treat COVID-19, with a specific focus on drug repurposing. medrxiv /10.1101/2020.05.27.20114371v1
A machine-readable version of this database can be downloaded here: Covid19db.txt. The ReDO database of repurposing candidates in oncology can be accessed here: ReDO_DB.
Summaries of results for Prevention and Therapeutic trials are listed here.
Important note for patients:This list includes trials of drugs that, based on their scientific properties, warrant further scientific investigation. In many cases the existing scientific evidence for their effects on coronavirus infection is very limited. Further scientific and clinical research is needed before any statements regarding their therapeutic activity can be made. This list is not intended to be used as a source for possible treatment options for patients.
If you need help exploring further treatment options for your situation, please contact your local medical services at the first instance.
| Summary results as of last build on: 20/08/2020 09:45 | |
| Total number of interventional trials - including non-drug trials (i.e. devices, psychological etc) | 2354 |
| Total number of interventional drug-based trials, including vaccines and cell/blood-based products, included in dataset | 1618 |
| Total number of investigational drugs included in trials | 550 |
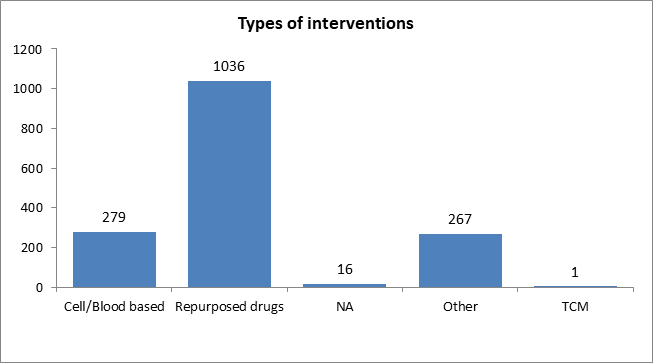
| Total number of trials including repurposed drugs | 1036 |
| Percentage of trials including repurposed drugs | 64.0% |
| Total number of prevention trials (vaccines + drugs) | 202 |
| Percentage of trials for prevention | 12.5% |
| Number of countries | 74 |
| Number of patients included in planned enrollment | 1064556 |
| Hydroxychloroquine | 212 | Favipiravir | 44 | Ivermectin | 39 |
| Azithromycin | 64 | Chloroquine | 43 | Methylprednisolone | 31 |
| Tocilizumab | 58 | Lopinavir/ritonavir | 43 | Ascorbic acid | 30 |

Click in header cell to sort table by that column. Hover over header cell for additional text.
Database build date: 20/08/2020 09:45
Number of trials in database: 1618 (Filter not active)
| ID | Title | Country | Ctl | MA | Type | Drugs | Primary Endpoint | Population |
|---|---|---|---|---|---|---|---|---|
| NCT04319900 | Clinical Trial of Favipiravir Tablets Combine With Chloroquine Phosphate in the Treatment of Novel Coronavirus Pneumonia | China | Y | Y | Drug Repurposing | Favipiravir, Chloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04252885 | The Efficacy of Lopinavir Plus Ritonavir and Arbidol Against Novel Coronavirus Infection | China | Y | Y | Drug Repurposing | Lopinavir/ritonavir, Umifenovir | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04282902 | A Study to Evaluate the Efficacy and Safety of Pirfenidone With Novel Coronavirus Infection | China | Y | N | Drug Repurposing | Pirfenidone | Efficacy - Oxygen parameters | Mild/Moderate Cases |
| NCT04329520 | EPICOS Clinical Trial for the Prevention of Infection in Healthcare Personnel | Spain | Y | Y | Drug Repurposing | Emtricitabine/tenofovir, Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| 2020-00122 4-33 |
Randomized controlled trial of hydroxychloroquine versus placebo for the treatment of adult patients with acute coronavirus disease 2019 – COVID-19 | Germany | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04343677 | Military COVID-19 Hydroxychloroquine Pre-exposure and Post-exposure Prophylaxis Study | United States | Y | Y | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| 2020-00159 1-15 |
Reducing hospital admission of elderly in SARS-CoV-2 pandemic via the induction of trained immunity by bacillus Calmette-Guérin vaccination, a randomized controlled trial. (COVID-19) | Netherlands | Y | N | Drug Repurposing | BCG Vaccine | Efficacy - Other efficacy | Other |
| NCT04380948 | A Pilot Study Evaluating the Effect of NT-I7, a Long Acting Interleukin-7, to Enhance Immune Clearance of SARS-CoV-2 (COVID-19) | United States | Y | N | Shelved drug or NME | NT-17 | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04308668 | Post-exposure Prophylaxis / Preemptive Therapy for SARS-Coronavirus-2 | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | General population |
| NCT04276987 | A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia | China | N | N | Cell/Blood based | ND:Stem cells | Safety | Severe Cases |
| 2020-00168 4-89 |
CATALYST - A randomised phase II proof of principle multi-arm multi-stage trial designed to guide the selection of interventions for phase III trials in hospitalised patients with COVID-19 infection. | United Kingdom | Y | N | Drug Repurposing | Gemtuzumab ozogamicin | Efficacy - Oxygen parameters | Severe Cases |
| NCT04380961 | A Study to Evaluate the Efficacy and Safety of Sirukumab in Confirmed Severe or Critical Confirmed Coronavirus Disease (COVID)-19 | United States | Y | N | Shelved drug or NME | Sirukumab | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04328467 | Pre-exposure Prophylaxis for SARS-Coronavirus-2 | United States | Y | Y | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | General population |
| NCT04333251 | Study Testing Convalescent Plasma vs Best Supportive Care | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04380701 | A Trial Investigating the Safety and Effects of Four BNT162 Vaccines Against COVID-2019 in Healthy Adults | Germany | N | N | Vaccine | ND:BNT162 vaccine | Safety | General population |
| NCT04367168 | Colchicine Twice Daily During 10 Days as an Option for the Treatment of Symptoms Induced by Inflammation in Patients With Mild and Severe Coronavirus Disease | Mexico | Y | N | Drug Repurposing | Colchicine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04316377 | Norwegian Coronavirus Disease 2019 Study | Norway | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04276688 | Lopinavir/ Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment | Hong Kong | Y | Y | Drug Repurposing | Ribavirin, Interferon beta-1b | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT03852537 | Steroid Dosing by bioMARker Guided Titration in Critically Ill Patients With Pneumonia | United States | Y | N | Drug Repurposing | Methylprednisolone | Other | Severe Cases |
| NCT04404192 | PH94B in the Treatment of Adjustment Disorder With Anxiety | United States | N | N | Shelved drug or NME | PH94B | Efficacy - Other efficacy | General population |
| NCT04356833 | Nebulised Rt-PA for ARDS Due to COVID-19 | United Kingdom | N | N | Shelved drug or NME | Nebulised Rt-PA | Efficacy - Oxygen parameters | Severe Cases |
| NCT04392219 | COVID-19 First In Human Study to Evaluate Safety, Tolerability, and Pharmacokinetics of EIDD-2801 in Healthy Volunteers | United States | Y | N | Shelved drug or NME | EIDD-2801 | Safety | General population |
| NCT04373707 | Weight-Adjusted vs Fixed Low Doses of Low Molecular Weight Heparin For Venous Thromboembolism Prevention in COVID-19 | France | Y | N | Drug Repurposing | Enoxaparin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT03648372 | A Study to Evaluate the Safety, Tolerability and Pharmacokinetics (PK) of TAK-981 in Adult Participants With Advanced or Metastatic Solid Tumors or Relapsed/Refractory Hematologic Malignancies and in a Subset With Coronavirus Disease 2019 (COVID-19) | United States | N | N | Shelved drug or NME | TAK-981 | Safety | Other |
| NCT04393415 | Using PRP and Cord Blood in Treatment of Covid -19 | Egypt | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04264533 | Vitamin C Infusion for the Treatment of Severe 2019-nCoV Infected Pneumonia | China | Y | N | Drug Repurposing | Ascorbic acid | Efficacy - Oxygen parameters | Severe Cases |
| NCT04323527 | Chloroquine Diphosphate for the Treatment of Severe Acute Respiratory Syndrome Secondary to SARS-CoV2 | Brazil | Y | N | Drug Repurposing | Chloroquine | Efficacy - Mortality | Severe Cases |
| NCT04322682 | Colchicine Coronavirus SARS-CoV2 Trial (COLCORONA) | Canada | Y | N | Drug Repurposing | Colchicine | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04385836 | Trial of Alpha One Antitrypsin Inhalation in Treating Patient With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) | Saudi Arabia | Y | N | Cell/Blood based | ND:Alpha-1 antitrypsin | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04275245 | Clinical Study of Anti-CD147 Humanized Meplazumab for Injection to Treat With 2019-nCoV Pneumonia | China | N | N | Shelved drug or NME | Meplazumab | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04341727 | Hydroxychloroquine,Hydroxychloroquine,Azithromycin in the Treatment of SARS CoV-2 Infection | United States | Y | Y | Drug Repurposing | Hydroxychloroquine, Azithromycin, Chloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04342728 | Coronavirus 2019 (COVID-19)- Using Ascorbic Acid and Zinc Supplementation | United States | Y | N | Drug Repurposing, Other | Ascorbic Acid, ND:Zinc Gluconate | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04404634 | Convalescent Plasma To Limit COVID-19 Associated Complications | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04347031 | A Study of the Effectiveness of an Off Label Mefloquine Use for the Treatment of Patients With COVID19 | Russian Federation | Y | Y | Drug Repurposing | Mefloquine, Hydroxychloroquine, Azithromycin, Tocilizumab | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04390152 | Safety and Efficacy of Intravenous Wharton's Jelly Derived Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome Due to COVID 19 | Colombia | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Mortality | Severe Cases |
| NCT04348370 | BCG Vaccine for Health Care Workers as Defense Against COVID 19 | United States | Y | N | Vaccine | BCG Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04343729 | Methylprednisolone in the Treatment of Patients With Signs of Severe Acute Respiratory Syndrome in Covid-19 | Brazil | Y | N | Drug Repurposing | Methylprednisolone | Efficacy - Mortality | Severe Cases |
| NCT04323800 | Convalescent Plasma to Stem Coronavirus (CSSC-001) | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - WHO scale | Exposed individuals (family members, HCP) |
| NCT04376034 | Convalescent Plasma Collection and Treatment in Pediatrics and Adults | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04269525 | Umbilical Cord(UC)-Derived Mesenchymal Stem Cells(MSCs) Treatment for the 2019-novel Coronavirus(nCOV) Pneumonia | China | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04261907 | Evaluating and Comparing the Safety and Efficiency of ASC09/Ritonavir and Lopinavir/Ritonavir for Novel Coronavirus Infection | China | Y | N | Drug Repurposing, Shelved drug or NME | ASC09/ritonavir | Efficacy - Oxygen parameters | Mild/Moderate Cases |
| NCT04340557 | Do Angiotensin Receptor Blockers Mitigate Progression to Acute Respiratory Distress Syndrome With SARS-CoV-2 Infection | United States | Y | N | Drug Repurposing | Losartan | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04323592 | Efficacy of Methylprednisolone for Patients With COVID-19 Severe Acute Respiratory Syndrome | Italy | N | N | Drug Repurposing | Methylprednisolone | Efficacy - Other efficacy | Severe Cases |
| NCT04345289 | Efficacy and Safety of Novel Treatment Options for Adults With COVID-19 Pneumonia | Denmark | Y | Y | Drug Repurposing, Cell/Blood based | ND:Convalescent plasma, Sarilumab, Baricitinib, Hydroxychloroquine | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04353037 | PATCH 2&3:Prevention & Treatment of COVID-19 (Severe Acute Respiratory Syndrome Coronavirus 2) With Hydroxychloroquine | United States | Y | Y | Drug Repurposing | Hydroxychloroquine | Efficacy - Other efficacy | Exposed individuals (family members, HCP), Mild/Moderate Cases, Severe Cases |
| NCT04280224 | NK Cells Treatment for COVID-19 | China | Y | N | Cell/Blood based | ND:T-cells | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04388826 | COVID-19 Treatment of Severe Acute Respiratory Syndrome With Veru-111 | United States | Y | N | Shelved drug or NME | Veru-111 | Efficacy - Other efficacy | Severe Cases |
| NCT04326426 | ODYSSEY: A Study to Investigate the Efficacy of Tradipitant in Treating Severe or Critical COVID-19 Infection | United States | Y | N | Shelved drug or NME | Tradipitant | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04299152 | Stem Cell Educator Therapy Treat the Viral Inflammation in COVID-19 | China | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Other efficacy | Severe Cases |
| NCT04306393 | Nitric Oxide Gas Inhalation in Severe Acute Respiratory Syndrome in COVID-19 | United States | Y | N | Drug Repurposing | Nitric oxide | Efficacy - Oxygen parameters | Severe Cases |
| NCT04361552 | Tocilizumab for the Treatment of Cytokine Release Syndrome in Patients With COVID-19 (SARS-CoV-2 Infection) | United States | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04322773 | Anti-il6 Treatment of Serious COVID-19 Disease With Threatening Respiratory Failure | Denmark | Y | Y | Drug Repurposing | Tocilizumab, Sarilumab | Efficacy - Oxygen parameters | Severe Cases |
| NCT04336410 | Safety, Tolerability and Immunogenicity of INO-4800 for COVID-19 in Healthy Volunteers | United States | N | N | Vaccine | INO-4800 | Safety | General population |
| NCT04338802 | Efficacy and Safety of Nintedanib in the Treatment of Pulmonary Fibrosis in Patients With Moderate to Severe COVID -19 | China | Y | N | Drug Repurposing | Nintedanib | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04379518 | Rintatolimod and IFN Alpha-2b for the Treatment of Mild or Moderate COVID-19 Infection in Cancer Patients | United States | N | N | Drug Repurposing | Rintatolimod, Interferon alpha-2b | Safety | Mild/Moderate Cases |
| NCT04374539 | Plasma Exchange in Patients With COVID-19 Disease and Invasive Mechanical Ventilation: a Randomized Controlled Trial | Spain | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04325893 | Hydroxychloroquine Versus Placebo in COVID-19 Patients at Risk for Severe Disease | France | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Mortality | Mild/Moderate Cases |
| NCT04322123 | Safety and Efficacy of Hydroxychloroquine Associated With Azithromycin in SARS-Cov-2 Virus (COVID-19) | Brazil | Y | Y | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04326036 | Use of cSVF Via IV Deployment for Residual Lung Damage After Symptomatic COVID-19 Infection | United States | N | N | Cell/Blood based | ND:Stem cells | Safety | Other |
| NCT04337918 | Nitric Oxide Releasing Solutions to Prevent and Treat Mild/Moderate COVID-19 Infection | Canada | N | N | Shelved drug or NME | Nitric Oxide Releasing Solution | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP), Mild/Moderate Cases |
| NCT04346693 | An Open Randomized Study of Dalargin Effectiveness in Patients With Severe and Critical Manifestations of SARS-COVID-19 | Russian Federation | Y | N | Shelved drug or NME | Dalargin | Efficacy - Viral load | Severe Cases |
| NCT04322396 | Proactive Prophylaxis With Azithromycin and hydroxyChloroquine in Hospitalized Patients With COVID-19 | Denmark | Y | Y | Drug Repurposing | Azithromycin, Hydroxychloroquine | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04415073 | A Phase 2 Study to Evaluate Axatilimab for Hospitalized Patients With Respiratory Involvement Secondary to COVID-19 | United States | Y | N | Shelved drug or NME | Axatilimab | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04355637 | Inhaled Corticosteroid Treatment of COVID19 Patients With Pneumonia | Spain | Y | N | Drug Repurposing | Budesonide | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04315480 | Tocilizumab for SARS-CoV2 (COVID-19) Severe Pneumonitis | Italy | N | N | Drug Repurposing | Tocilizumab | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04399798 | Baricitinib for coRona Virus pnEumonia (COVID-19): a THerapeutic Trial | Italy | N | N | Drug Repurposing | Baricitinib | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04363437 | COlchicine in Moderate-severe Hospitalized Patients Before ARDS to Treat COVID-19 | United States | Y | N | Drug Repurposing | Colchicine | Efficacy - Oxygen parameters | Severe Cases |
| NCT04406246 | Prevention of Coronavirus Disease (COVID-19) Outbreaks With Nitazoxanide | Mexico | N | N | Drug Repurposing | Nitazoxanide | Efficacy - Other efficacy | Exposed individuals (family members, HCP) |
| NCT04341493 | Hydroxychloroquine vs Nitazoxanide in Patients With COVID-19 | Mexico | Y | N | Drug Repurposing | Nitazoxanide | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04322344 | Escin in Patients With Covid-19 Infection | Italy | Y | Y | Drug Repurposing | Escin | Efficacy - Mortality | Mild/Moderate Cases |
| NCT04344041 | COvid-19 and Vitamin D Supplementation: a Multicenter Randomized Controlled Trial of High Dose Versus Standard Dose Vitamin D3 in High-risk COVID-19 Patients (CoVitTrial) | France | Y | N | Drug Repurposing | Vitamin D3 | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases, Other |
| RBR-3cbs3w | Randomized, pragmatic, open study evaluating Hydroxychloroquine for prevention of Hospitalization and Respiratory Complications in outpatients with confirmed or presumptive diagnosis of Infection by (COVID-19) | Brazil | Y | Y | Drug Repurposing | Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04384458 | COVID-19 Prophylaxis With Hydroxychloroquine Associated With Zinc For High-Risk Healthcare Workers | Brazil | N | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04403685 | Safety and Efficacy of Tocilizumab in Moderate to Severe COVID-19 With Inflammatory Markers | Brazil | Y | N | Drug Repurposing | Tocilizumab | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04382768 | Inhaled Ibuprofen to Treat COVID-19 | Argentina | N | N | Drug Repurposing | Ibuprofen | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04414631 | Conestat Alfa in the Prevention of Severe SARS-CoV-2 Infection in Hospitalized Patients With COVID-19 | Switzerland | Y | N | Drug Repurposing | Conestat alfa | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04368377 | Enhanced Platelet Inhibition in Critically Ill Patients With COVID-19 | Italy | N | N | Drug Repurposing | Tirofiban, Clopidogrel, Aspirin, Fondaparinux | Efficacy - Oxygen parameters | Severe Cases |
| NCT04365309 | Protective Effect of Aspirin on COVID-19 Patients | China | Y | N | Drug Repurposing | Aspririn | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04312243 | NO Prevention of COVID-19 for Healthcare Providers | United States | Y | N | Drug Repurposing | Nitric oxide | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04406064 | Viral Specific T-cells for Treatment of COVID-19 | United States | N | N | Cell/Blood based | ND:T-cells | Other | Mild/Moderate Cases, Severe Cases |
| NCT04357613 | IMATINIB IN COVID-19 DISEASE IN AGED PATIENTS. | France | Y | N | Drug Repurposing | Imatinib | Safety | Other |
| NCT04363840 | The LEAD COVID-19 Trial: Low-risk, Early Aspirin and Vitamin D to Reduce COVID-19 Hospitalizations | United States | Y | N | Drug Repurposing | Aspirin, Vitamin D3 | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04371523 | Hydroxychloroquine to Prevent COVID-19 Disease Amongst Healthcare Workers | Canada | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04333654 | Hydroxychloroquine in Outpatient Adults With COVID-19 | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04345887 | Spironolactone in Covid-19 Induced ARDS | Turkey | Y | N | Drug Repurposing | Spironolactone | Efficacy - Oxygen parameters | Severe Cases |
| NCT04358783 | Convalescent Plasma Compared to the Best Available Therapy for the Treatment of SARS-CoV-2 Pneumonia | Mexico | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases |
| 2020-00143 5-27 |
Home treatment of elderly patients with symptomatic SARS-CoV-2 infection (COVID-19) : a multiarm, multi-stage (MAMS) randomized trial to assess the efficacy and safety of several experimental treatments to reduce the risk of hospitalization or death (COVERAGE trial) | France | Y | Y | Drug Repurposing | Favipiravir, Hydroxychloroquine, Imatinib, Telmisartan | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04334629 | LIBERATE Trial in COVID-19 | United Kingdom | Y | N | Drug Repurposing | Ibuprofen | Efficacy - Oxygen parameters | Severe Cases |
| NCT04324463 | Anti-Coronavirus Therapies to Prevent Progression of Coronavirus Disease 2019 (COVID-19) Trial | Canada | Y | N | Drug Repurposing | Cholchicine, Interferon-beta, Aspirin, Rivaroxaban | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04377672 | Human Convalescent Plasma for High Risk Children Exposed or Infected With SARS-CoV-2 (COVID-19) | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Safety | Other |
| 2020-00244 8-21 |
A RANDOMIZED CLINICAL TRIAL FOR ENHANCED TRAINED IMMUNE RESPONSES THROUGH BACILLUS CALMETTE-GUÉRIN VACCINATION TO PREVENT INFECTIONS ?? COVID-19: THE ACTIVATE II TRIAL | Greece | Y | N | Vaccine | ND:BCG vaccine | Efficacy - Infection rate (prevention) | General population |
| NCT04418531 | Convalescent Antibodies Infusion in COVID 19 Patients | Italy | N | N | Cell/Blood based | ND:Immunoglobulin | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04321278 | Safety and Efficacy of Hydroxychloroquine Associated With Azithromycin in SARS-CoV2 Virus (Coalition Covid-19 Brasil II) | Brazil | Y | Y | Drug Repurposing | Azithromycin, Hydroxychloroquine | Efficacy - WHO scale | Severe Cases |
| NCT04345614 | A Study of CM4620-Injectable Emulsion (IE) in Patients With Severe COVID-19 Pneumonia | United States | Y | N | Shelved drug or NME | CM4620 | Efficacy - WHO scale | Severe Cases |
| NCT04321174 | COVID-19 Ring-based Prevention Trial With Lopinavir/Ritonavir | Canada | Y | N | Drug Repurposing | Lopinavir/ritonavir | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04376684 | Investigating Otilimab in Patients With Severe Pulmonary COVID-19 Related Disease | United States | Y | N | Shelved drug or NME | Otilimab | Efficacy - Mortality | Severe Cases |
| NCT04393792 | SINUS WASH Pilot Study in Adults Testing Positive for COVID-19 | United Kingdom | Y | N | Shelved drug or NME | Povidone-Iodine | Efficacy - Viral load | Mild/Moderate Cases |
| NCT02735707 | Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community- Acquired Pneumonia | Netherlands | Y | Y | Drug Repurposing | Hydrocortisone, Ceftriaxone, Moxifloxacin, Levofloxacin, Piperacillin-tazobactam, Ceftaroline, Amoxicillin-clavulanate, Oseltamivir, Lopinavir/ritonavir, Hydroxychloroquine, Interferon-beta1a, Anakinra, Tocilizumab, Sarilumab | Efficacy - Mortality | Severe Cases |
| NCT04293887 | Efficacy and Safety of IFN-a2ß in the Treatment of Novel Coronavirus Patients | China | Y | N | Drug Repurposing | Interferon alpha-1b | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04327401 | COVID-19-associated ARDS Treated With Dexamethasone: Alliance Covid-19 Brasil III | Brazil | Y | N | Drug Repurposing | Dexamethasone | Efficacy - Other efficacy | Severe Cases |
| NCT04342156 | Safety And Efficacy Of Hydroxychloroquine For At Risk Population (SHARP) Against COVID-19 | Singapore | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04389385 | COVID-19 Specific T Cell Derived Exosomes (CSTC-Exo) | Turkey | N | N | Cell/Blood based | COVID-19 Specific T Cell derived exosomes | Safety | Mild/Moderate Cases |
| NCT04387409 | Study to Assess VPM1002 in Reducing Healthcare Professionals' Absenteeism in COVID-19 Pandemic | Germany | Y | N | Vaccine | ND:VPM1002 vaccine | Efficacy - Other efficacy | Exposed individuals (family members, HCP) |
| NCT04371601 | Safety and Effectiveness of Mesenchymal Stem Cells in the Treatment of Pneumonia of Coronavirus Disease 2019 | China | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Oxygen parameters | Severe Cases |
| NCT04260594 | Clinical Study of Arbidol Hydrochloride Tablets in the Treatment of Pneumonia Caused by Novel Coronavirus | China | Y | N | Drug Repurposing | Umifenovir | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04336748 | HCQ for Primary Prophylaxis Against COVID19 in Health-care Workers | Austria | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04383717 | Levamisole and Isoprinosine in the Treatment of COVID19: A Proposed Therapeutic Trial | Egypt | Y | N | Drug Repurposing | Levamisole, Isoprinosine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04429555 | Efficacy, Safety, Tolerability, and Biomarkers of MN-166 (Ibudilast) in Patients Hospitalized With COVID-19 and at Risk for ARDS | United States | Y | N | Drug Repurposing | Ibudilast | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04346589 | Convalescent Antibodies Infusion in Critically Ill COVID 19 Patients | Italy | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Severe Cases |
| NCT04340349 | Low-dose Hydroxychloroquine and Bromhexine: a Novel Regimen for COVID-19 Prophylaxis in Healthcare Professionals | Mexico | Y | N | Drug Repurposing | Hydroxychloroquine, Bromhexine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04439006 | Ibrutinib for the Treatment of COVID-19 in Patients Requiring Hospitalization | United States | Y | N | Drug Repurposing | Ibrutinib | Efficacy - Oxygen parameters | Other |
| NCT03042143 | Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration (REALIST) (COVID-19) | United Kingdom | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Oxygen parameters | Severe Cases |
| NCT04359810 | Plasma Therapy of COVID-19 in Critically Ill Patients | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04252274 | Efficacy and Safety of Darunavir and Cobicistat for Treatment of COVID-19 | China | Y | N | Drug Repurposing | Darunavir, Cobicistat | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04385186 | Inactivated Convalescent Plasma as a Therapeutic Alternative in Patients CoViD-19 | Colombia | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04305457 | Nitric Oxide Gas Inhalation Therapy for Mild/Moderate COVID-19 | United States | Y | N | Drug Repurposing | Nitric oxide | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04320277 | Baricitinib in Symptomatic Patients Infected by COVID-19: an Open-label, Pilot Study. | Italy | N | N | Drug Repurposing | Baricitinib | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04397497 | Mavrilimumab in Severe COVID-19 Pneumonia and Hyper-inflammation (COMBAT-19) | Italy | Y | N | Shelved drug or NME | Mavrilimumab | Efficacy - Oxygen parameters | Severe Cases |
| NCT04369794 | COVID-19: BCG As Therapeutic Vaccine, Transmission Limitation, and Immunoglobulin Enhancement | Brazil | Y | N | Vaccine | BCG vaccine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04353128 | Efficacy of Melatonin in the Prophylaxis of Coronavirus Disease 2019 (COVID-19) Among Healthcare Workers. | Spain | Y | N | Drug Repurposing | Melatonin | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04365985 | Study of Immunomodulation Using Naltrexone and Ketamine for COVID-19 | United States | Y | Y | Drug Repurposing | Naltrexone, Ketamine | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04352946 | HEalth Care Worker pROphylaxis Against COVID-19: The HERO Trial | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04331613 | Safety and Efficacy of CAStem for Severe COVID-19 Associated With/Without ARDS | China | N | N | Cell/Blood based | ND:Stem cells | Safety | Severe Cases |
| NCT04334967 | Hydroxychloroquine in Patients With Newly Diagnosed COVID-19 Compared to Standard of Care | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04363827 | Protect: Study With Hydroxychloroquine for Prevention and Early Phase Treatment of Coronavirus Disease (COVID-19) | Italy | Y | Y | Drug Repurposing | Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04340050 | COVID-19 Convalescent Plasma | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Other | Severe Cases |
| NCT04363450 | Hydroxychloroquine as Prophylaxis for COVID-19 in Healthcare Workers (HCQPreP) | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04335786 | Valsartan for Prevention of Acute Respiratory Distress Syndrome in Hospitalized Patients With SARS-COV-2 (COVID-19) Infection Disease | Netherlands | Y | N | Drug Repurposing | Valsartan | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04446429 | Anti-Androgen Treatment for COVID-19 | Brazil | Y | N | Drug Repurposing | Dutasteride | Efficacy - Clinical improvement (except WHO scale) | Other |
| NCT04321616 | The Efficacy of Different Anti-viral Drugs in COVID 19 Infected Patients | Norway | Y | Y | Drug Repurposing, Shelved drug or NME | Hydroxychloroquine, Remdesivir | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04263402 | The Efficacy of Different Hormone Doses in 2019-nCoV Severe Pneumonia | China | N | Y | Drug Repurposing | Methylprednisolone | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04392778 | Clinical Use of Stem Cells for the Treatment of Covid-19 | Turkey | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases, Other |
| NCT04407182 | Safety and Efficacy of Viusid and Asbrip in Hospitalized Patients Infected by SARS-Cov-2 With COVID-19 | Ecuador | Y | N | Shelved drug or NME | Viusid/Asbrip | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04424134 | BromhexIne And Spironolactone For Coron?VirUs Infection Requiring HospiTalization | Russian Federation | Y | N | Drug Repurposing | Bromhexine, Spironolactone | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04332666 | Angiotensin-(1,7) Treatment in COVID-19: the ATCO Trial | Belgium | Y | N | Drug Repurposing | Angiotensin 1-7 | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04332991 | Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04397692 | Inhaled NO for the Treatment of COVID-19 Caused by SARS-CoV-2 (US Trial) | United States | Y | N | Drug Repurposing | Nitric oxide | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04433078 | RepurpoSing Old Drugs TO SuppRess a Modern Threat: COVID-19 STORM | United States | Y | N | Drug Repurposing | Doxycycline | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04382547 | Treatment of Covid-19 Associated Pneumonia With Allogenic Pooled Olfactory Mucosa-derived Mesenchymal Stem Cells | Belarus | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04374565 | Convalescent Plasma for Treatment of COVID-19 Patients With Pneumonia | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04298060 | DAS181 for Patients With Severe Hospitalized Flu and SAD-RVs (COVID-19) | China | Y | Y | Shelved drug or NME | DAS181 | Efficacy - Oxygen parameters | Severe Cases |
| NCT04343651 | Study to Evaluate the Efficacy and Safety of Leronlimab for Mild to Moderate COVID-19 | United States | Y | N | Shelved drug or NME | Leronlimab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04384380 | Efficacy and Tolerability of Hydroxychloroquine in Adult Patients With COVID-19 | Taiwan | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04381364 | Inhalation of Ciclesonide for Patients With COVID-19: A Randomised Open Treatment Study (HALT COVID-19) | Sweden | Y | N | Drug Repurposing | Ciclesonide | Efficacy - Oxygen parameters | Mild/Moderate Cases |
| NCT04321096 | The Impact of Camostat Mesilate on COVID-19 Infection | Denmark | Y | N | Drug Repurposing | Camostat | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04355962 | Sevoflurane in COVID-19 ARDS (SevCov) | Switzerland | Y | N | Drug Repurposing | Sevoflurane | Efficacy - Mortality | Severe Cases |
| NCT04333368 | Cell Therapy Using Umbilical Cord-derived Mesenchymal Stromal Cells in SARS-CoV-2-related ARDS | France | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Oxygen parameters | Severe Cases |
| NCT04416399 | STerOids in COVID-19 Study | United Kingdom | Y | N | Drug Repurposing | Budesonide | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04381377 | Efficacy and Safety of Polyoxidonium® in Hospitalized Patients With Coronavirus Disease COVID-19 | Russian Federation | Y | N | Drug Repurposing | Azoximer bromide | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04349228 | Assessment of the Efficacy and Safety of (HCQ) as a Prophylaxis for COVID19 for Health Professionals | Tunisia | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04347239 | Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19) | United States | Y | N | Shelved drug or NME | Leronlimab | Efficacy - Mortality | Severe Cases |
| NCT04403100 | Hydroxychloroquine and Lopinavir/ Ritonavir to Improve the Health of People With COVID-19: "The Hope Coalition - 1" | Brazil | Y | N | Drug Repurposing | Hydroxychloroquine, Lopinavir/Ritonavir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04403243 | COLchicine Versus Ruxolitinib and Secukinumab In Open Prospective Randomized Trial | Russian Federation | Y | Y | Drug Repurposing | Colchicine, Ruxolitinib, Secukinumab | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04442230 | NasoVAX in Patients With Early Coronavirus Infectious Disease 2019 (COVID-19) | United States | Y | N | Vaccine | ND:NasoVAX | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04361474 | Trial Evaluating the Efficacy of Local Budesonide Therapy in the Management of Hyposmia in COVID-19 Patients Without Signs of Severity | France | Y | N | Drug Repurposing | Budesonide | Other | Mild/Moderate Cases |
| NCT04402957 | LSALT Peptide vs. Placebo to Prevent ARDS and Acute Kidney Injury in Patients Infected With SARS-CoV-2 (COVID-19) | Canada | Y | N | Shelved drug or NME | LSALT peptide | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04448756 | M5049 Study in Participants With Coronavirus Disease 2019 (COVID-19) Pneumonia | United States | Y | N | Shelved drug or NME | M5049 | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04349098 | Evaluation of Activity and Safety of Oral Selinexor in Participants With Severe COVID-19 Infection | United States | Y | N | Drug Repurposing | Selinexor | Efficacy - WHO scale | Severe Cases |
| NCT04332380 | Convalescent Plasma for Patients With COVID-19: A Pilot Study | Colombia | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04355676 | Evaluation of Activity and Safety of Two Regimens of Low Dose Oral Selinexor in Participants With Moderate or Severe COVID-19 | United States | N | N | Drug Repurposing | Selinexor | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04395716 | A Study of ResCure™ to Treat COVID-19 Infection | United States | N | N | Shelved drug or NME | ResCure | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04419623 | A Study of TL-895 With Standard Available Treatment Versus Standard Available Treatment for the Treatment of COVID-19 in Patients With Cancer | United States | Y | N | Shelved drug or NME | TL-895 | Other | Other |
| NCT04437823 | Efficacy of Intravenous Infusions of Stem Cells in the Treatment of COVID-19 Patients | Pakistan | Y | N | Cell/Blood based | ND:Stem cells | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04402060 | A Study of APL-9 in Adults With Mild to Moderate ARDS Due to COVID-19 | United States | Y | N | Shelved drug or NME | APL-9 | Safety | Mild/Moderate Cases |
| NCT04371978 | Efficacy and Safety of Dipeptidyl Peptidase-4 Inhibitors in Diabetic Patients With Established COVID-19 | Israel | Y | N | Drug Repurposing | Linagliptin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Other |
| NCT04332835 | Convalescent Plasma for Patients With COVID-19: A Randomized, Open Label, Parallel, Controlled Clinical Study | Colombia | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04312009 | Losartan for Patients With COVID-19 Requiring Hospitalization | United States | Y | N | Drug Repurposing | Losartan | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases, Mild/Moderate Cases, Mild/Moderate Cases |
| NCT04315948 | Trial of Treatments for COVID-19 in Hospitalized Adults | France | Y | Y | Drug Repurposing, Shelved drug or NME | Remdesivir, Lopinavir/ritonavir, Interferon beta-1a, Hydroxychloroquine | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04311177 | Losartan for Patients With COVID-19 Not Requiring Hospitalization | United States | Y | N | Drug Repurposing | Losartan | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04402970 | Dornase Alfa for ARDS in Patients With SARS-CoV-2 | United States | N | N | Drug Repurposing | Dornase alfa | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04335084 | A Study of Hydroxychloroquine, Vitamin C, Vitamin D, and Zinc for the Prevention of COVID-19 Infection | United States | N | N | Drug Repurposing | Hydroxychloroquine, Ascorbic acid, Vitamin D3, Zinc | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04264858 | Treatment of Acute Severe 2019-nCoV Pneumonia With Immunoglobulin From Cured Patients | China | Y | N | Cell/Blood based | ND:Immunoglobulin | Efficacy - WHO scale | Severe Cases |
| NCT04322565 | Colchicine Counteracting Inflammation in COVID-19 Pneumonia | Italy | Y | N | Drug Repurposing | Colchicine | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04341935 | Effects of DPP4 Inhibition on COVID-19 | United States | Y | N | Drug Repurposing | Linagliptin | Other | Mild/Moderate Cases |
| NCT04367077 | MultiStem Administration for COVID-19 Induced ARDS (MACoVIA) | United States | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Other efficacy | Severe Cases |
| NCT04365101 | Natural Killer Cell (CYNK-001) Infusions in Adults With COVID-19 (CYNK-001-COVID-19) | United States | Y | N | Cell/Blood based | ND:CYNK-001 | Safety | Mild/Moderate Cases |
| NCT04370834 | Tocilizumab for Patients With Cancer and COVID-19 Disease | United States | N | N | Drug Repurposing | Tocilizumab | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases, Other |
| NCT04275414 | Bevacizumab in Severe or Critical Patients With COVID-19 Pneumonia | China | N | N | Drug Repurposing | Bevacizumab | Efficacy - Oxygen parameters | Severe Cases |
| NCT04421664 | Preemptive Therapy for SARS-Coronavirus-2 (COVID-19 PEP Canada) | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04360096 | Inhaled Aviptadil for the Treatment of Moderate and Severe COVID-19 | United States | Y | N | Shelved drug or NME, Other | Aviptadil, ND:Device | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04342169 | University of Utah COVID-19 Hydrochloroquine Trial | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04320238 | Experimental Trial of rhIFNa Nasal Drops to Prevent 2019-nCOV in Medical Staff | China | N | Y | Drug Repurposing | Interferon alpha, Thymosin alpha | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04327349 | Investigating Effect of Convalescent Plasma on COVID-19 Patients Outcome: A Clinical Trial | Iran | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04400019 | Prevention of COVID19 Infection in Nursing Homes by Chemoprophylaxis With Hydroxychloroquine (PREVICHARM) | Spain | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Other |
| NCT04433546 | PB1046, a Long-acting, Sustained Release Human VIP Analogue, Intended to Provide Clinical Improvement to Hospitalized COVID-19 Patients at High Risk for Rapid Clinical Deterioration and Acute Respiratory Distress Syndrome (ARDS). | United States | Y | N | Shelved drug or NME | PB1046 | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04357730 | Fibrinolytic Therapy to Treat ARDS in the Setting of COVID-19 Infection | United States | Y | N | Drug Repurposing | Alteplase | Efficacy - Oxygen parameters | Severe Cases |
| NCT04407208 | Convalescent Plasma Therapy in Patients With COVID-19 | Indonesia | N | N | Cell/Blood based | ND:Convalescent plasma | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04394377 | Full Anticoagulation Versus Prophylaxis in COVID-19: COALIZAO ACTION Trial | Brazil | Y | N | Drug Repurposing | Rivaroxaban | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04453371 | Impact of Tissue Plasminogen Activator (tPA) Treatment for an Atypical Acute Respiratory Distress Syndrome (COVID-19) | Russian Federation | Y | N | Drug Repurposing | Alteplase | Efficacy - Oxygen parameters | Severe Cases |
| NCT04342650 | Chloroquine Diphosphate in the Prevention of SARS in Covid-19 Infection | Brazil | Y | N | Drug Repurposing | Chloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04452565 | NA-83, Atazanavir and Dexamethasone Combination Therapy for the Treatment of COVID-19 Infection | United States | Y | Y | Drug Repurposing | Atazanavir, Dexamethasone | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04324606 | A Study of a Candidate COVID-19 Vaccine (COV001) | United Kingdom | N | Y | Vaccine | ND:Vaccine, Paracetamol | Safety | General population |
| NCT04373733 | Early Intervention in COVID-19: Favipiravir Verses Standard Care | United Kingdom | Y | Y | Drug Repurposing | Favipiravir | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04459702 | A Study of Combination Therapies to Treat COVID-19 Infection | United States | Y | Y | Drug Repurposing | Lopinavir, Ritonavir, Azithromycin, Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04400838 | Investigating a Vaccine Against COVID-19 | United Kingdom | Y | Y | Vaccine | ND:Vaccine | Efficacy - Infection rate (prevention) | General population |
| NCT04344379 | Prevention of SARS-CoV-2 in Hospital Workers s Exposed to the Virus | France | Y | Y | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04268537 | Immunoregulatory Therapy for 2019-nCoV | China | Y | Y | Drug Repurposing | Anti-PD-1(NOS), Thymosin alpha | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04355052 | Open Label Study to Compare Efficacy, Safety and Tolerability of Hydroxychloroquine Combined With Azithromycin Compared to Hydroxychloroquine Combined With Camostat Mesylate and to "no Treatment" in SARS CoV 2 Virus | Israel | Y | Y | Drug Repurposing | Azithromycin, Camostat | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04330495 | Randomized, Controlled, Double-blind Clinical Trial Comparing the Efficacy and Safety of Chemoprophylaxis With Hydroxychloroquine in Patients Under Biological Treatment and / or JAK Inhibitors in the Prevention of SARS-CoV-2 Infection | Spain | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Other |
| NCT04447781 | Safety, Tolerability and Immunogenicity of INO-4800 Followed by Electroporation in Healthy Volunteers for COVID19 | Korea, Republic of | Y | Y | Vaccine, Other | ND:Vaccine INO-4800, ND:Device | Safety | Other |
| NCT04447235 | Early Treatment With Ivermectin and LosarTAN for Cancer Patients With COVID-19 Infection | Brazil | Y | N | Drug Repurposing | Losartan, Ivermectin | Efficacy - Clinical improvement (except WHO scale) | Other |
| NCT04405739 | The Safety of EIDD-2801 and Its Effect on Viral Shedding of SARS-CoV-2 (The END-COVID Study) | United States | Y | Y | Shelved drug or NME | EIDD-2801 | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04447404 | DUR-928 in Subjects With SARS-CoV-2 With Acute Liver or Kidney Injury | United States | Y | N | Shelved drug or NME | DUR-928 | Efficacy - Mortality | Other |
| NCT04261426 | The Efficacy of Intravenous Immunoglobulin Therapy for Severe 2019-nCoV Infected Pneumonia | China | N | N | Cell/Blood based | ND:Immunoglobulin | Efficacy - WHO scale | Severe Cases |
| NCT04386070 | Preventing Pulmonary Complications in Surgical Patients at Risk of COVID-19 | United Kingdom | Y | Y | Drug Repurposing | Lopinavir-Ritonavir, Hydroxychloroquine | Efficacy - Infection rate (prevention) | Other |
| NCT04255017 | A Prospective/Retrospective,Randomized Controlled Clinical Study of Antiviral Therapy in the 2019-nCoV Pneumonia | China | Y | Y | Drug Repurposing | Oseltamivir, Lopinavir/ritonavir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04254874 | A Prospective/Retrospective,Randomized Controlled Clinical Study of Interferon Atomization in the 2019-nCoV Pneumonia | China | Y | N | Drug Repurposing | Peg-Interferon alpha-2b | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04408235 | High Versus Low LMWH Dosages in Hospitalized Patients With Severe COVID-19 Pneumonia and Coagulopathy | Italy | Y | N | Drug Repurposing | Enoxaparin | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04261270 | A Randomized,Open,Controlled Clinical Study to Evaluate the Efficacy of ASC09F and Ritonavir for 2019-nCoV Pneumonia | China | N | Y | Drug Repurposing, Shelved drug or NME | Ritonavir, Oseltamivir, ASC09F | Efficacy - Oxygen parameters | Mild/Moderate Cases |
| NCT04350281 | Double Therapy With IFN-beta 1b and Hydroxychloroquine | Hong Kong | Y | N | Drug Repurposing | Interferon beta-1b | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04332042 | TOFAcitinib in SARS-CoV2 Pneumonia | Italy | Y | N | Drug Repurposing | Tofacitinib | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04371393 | MSCs in COVID-19 ARDS | United States | Y | N | Cell/Blood based | ND:Remestemcel-L® | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04467918 | CANnabiDiol for CoviD-19 pATiEnts With Mild to Moderate Symptoms | Brazil | Y | N | Drug Repurposing | Cannabidiol | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04357366 | suPAR-guided Anakinra Treatment for Validation of the Risk and Management of Respiratory Failure by COVID-19 (SAVE) | Greece | N | N | Drug Repurposing | Anakinra, Trimethoprim/sulfamethoxazole | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04346667 | Post-Exposure Prophylaxis for Asymptomatic SARS-CoV-2 COVID-19 Patients With choloroquinE Compounds | Pakistan | Y | Y | Drug Repurposing | Hydroxychloroquine, Chloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04355429 | Efficacy of Captopril in Covid-19 Patients With Severe Acute Respiratory Syndrome (SARS) CoV-2 Pneumonia (CAPTOCOVID) | France | Y | N | Drug Repurposing | Captopril | Efficacy - Other efficacy | Severe Cases |
| NCT04420247 | Efficacy of Chloroquine or Hydroxychloroquine in Treating Pneumonia Caused by SARS-Cov-2 - COVID-19 | Brazil | Y | N | Drug Repurposing | Hydroxychloroquine, Chloroquine | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04356482 | CONVALESCENT PLASMA FOR ILL PATIENTS BY COVID-19 | Mexico | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04311697 | Intravenous Aviptadil for Critical COVID-19 With Respiratory Failure | United States | Y | N | Shelved drug or NME | Aviptadil | Efficacy - Mortality | Severe Cases |
| NCT04419025 | Efficacy of N-Acetylcysteine (NAC) in Preventing COVID-19 From Progressing to Severe Disease | United States | Y | N | Drug Repurposing | N-acetylcysteine | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04407507 | Efficacy, Safety and Tolerability of Ivermectin in Subjects Infected With SARS-CoV-2 With or Without Symptoms | Mexico | Y | N | Drug Repurposing | Ivermectin | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04479644 | Safety, Tolerability, and Pharmacokinetics Study of Human Monoclonal Antibody BRII-198 | China | Y | N | Shelved drug or NME | BRII-198 | Safety | Other |
| NCT04479631 | Safety, Tolerability, and Pharmacokinetics Study of Human Monoclonal Antibody BRII-196 | China | Y | N | Shelved drug or NME | BRII-196 | Safety | Other |
| NCT04357782 | Administration of Intravenous Vitamin C in Novel Coronavirus Infection (COVID-19) and Decreased Oxygenation | United States | N | N | Drug Repurposing | Ascorbic acid | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04400799 | Enoxaparin for Primary Thromboprophylaxis in Ambulatory Patients With COVID-19 | Switzerland | Y | N | Drug Repurposing | Enoxaparin | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04344184 | Early Infusion of Vitamin C for Treatment of Novel COVID-19 Acute Lung Injury (EVICT-CORONA-ALI) | United States | Y | N | Drug Repurposing | Ascorbic acid | Efficacy - Other efficacy | Severe Cases |
| NCT04378244 | CORONA: A Study Using DeltaRex-G Gene Therapy for Symptomatic COVID-19 | United States | N | N | Shelved drug or NME | DeltaRex-G | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04333914 | Prospective Study in Patients With Advanced or Metastatic Cancer and SARS-CoV-2 Infection | France | Y | Y | Drug Repurposing, Shelved drug or NME | GNS651, Nivolumab, Tocilizumab, Avdoralimab, Monalizumab | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04398147 | Phase I/II Clinical Trial of Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) in Canada | Canada | Y | Y | Vaccine | ND:Ad5-nCoV | Safety | General population |
| NCT04327206 | BCG Vaccination to Protect Healthcare Workers Against COVID-19 | Australia | Y | N | Drug Repurposing, Vaccine | BCG Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04466098 | Multiple Dosing of Mesenchymal Stromal Cells in Patients With ARDS (COVID-19) | United States | Y | N | Cell/Blood based | ND:MSC | Safety | Severe Cases |
| NCT04334512 | A Study of Quintuple Therapy to Treat COVID-19 Infection | United States | N | N | Drug Repurposing | Hydroxychloroquine, Azithromycin, Ascorbic acid, Vitamin D3, Zinc | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04328961 | Hydroxychloroquine for COVID-19 Post-exposure Prophylaxis (PEP) | United States | Y | Y | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | General population |
| NCT04425733 | MK-5475 in Participants With Hypoxemia Due to COVID-19 Pneumonia (MK-5475-009) | United States | Y | N | Shelved drug or NME | MK-5475 | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04461379 | Prevention, Efficacy and Safety of BCG Vaccine in COVID-19 Among Healthcare Workers | Mexico | Y | N | Vaccine | ND:Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04392232 | A Study of COVID 19 Convalescent Plasma in High Risk Patients With COVID 19 Infection | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| NCT04339712 | Personalised Immunotherapy for SARS-CoV-2 (COVID-19) Associated With Organ Dysfunction | Greece | N | N | Drug Repurposing | Anakinra, Tocilizumab | Efficacy - Other efficacy | Severe Cases |
| NCT04455958 | Lopinavir/Ritonavir for the Treatment of COVID-19 Positive Patients With Cancer and Immune Suppression in the Last Year | United States | Y | N | Drug Repurposing | Lopinavir/Ritonavir | Efficacy - Clinical improvement (except WHO scale) | Other |
| NCT04389580 | Combination Therapy With Isotretinoin and Tamoxifen Expected to Provide Complete Protection Against Severe Acute Respiratory Syndrome Coronavirus | Egypt | Y | Y | Drug Repurposing | Isotretinoin, Tamoxifen | Efficacy - Other efficacy | Severe Cases |
| NCT04357106 | COPLA Study: Treatment of Severe Forms of COronavirus Infection With Convalescent PLAsma | Mexico | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Severe Cases |
| NCT04334148 | Healthcare Worker Exposure Response and Outcomes of Hydroxychloroquine | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04333589 | Corona Virus Disease 2019 Patients Whose Nucleic Acids Changed From Negative to Positive | China | Y | N | Drug Repurposing | Favipiravir | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04310228 | Favipiravir Combined With Tocilizumab in the Treatment of Corona Virus Disease 2019 | China | Y | Y | Drug Repurposing | Favipiravir, Tocilizumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04302519 | Novel Coronavirus Induced Severe Pneumonia Treated by Dental Pulp Mesenchymal Stem Cells | China | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Other efficacy | Severe Cases |
| NCT04370015 | Hydroxychloroquine Chemoprophylaxis for COVID-19 Infection in High-risk Healthcare Workers. | Pakistan | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04435015 | The Utility of Camostat Mesylate in Patients With COVID-19 Associated Coagulopathy (CAC) and Cardiovascular Complications | United States | Y | N | Drug Repurposing | Camostat | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04391179 | Dipyridamole to Prevent Coronavirus Exacerbation of Respiratory Status (DICER) in COVID-19 | United States | Y | N | Drug Repurposing | Dipyridamole | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04273763 | Evaluating the Efficacy and Safety of Bromhexine Hydrochloride Tablets Combined With Standard Treatment/ Standard Treatment in Patients With Suspected and Mild Novel Coronavirus Pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Bromhexine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04393727 | Transfusion of Convalescent Plasma for the Early Treatment of Patients With COVID-19 | Italy | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04490486 | Umbilical Cord Tissue (UC) Derived Mesenchymal Stem Cells (MSCs) Versus Placebo to Treat Acute Pulmonary Inflammation Due to COVID-19 | United States | Y | N | Cell/Blood based | ND:Stem cells | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04354155 | COVID-19 Anticoagulation in Children - Thromboprophlaxis (COVAC-TP) Trial | United States | N | N | Drug Repurposing | Enoxaparin | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04435587 | Ivermectin vs Combined Hydroxychloroquine and Antiretroviral Drugs (ART) Among Asymptomatic COVID-19 Infection | Thailand | Y | N | Drug Repurposing | Ivermectin | Safety | Mild/Moderate Cases |
| NCT04341441 | Will Hydroxychloroquine Impede or Prevent COVID-19 | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04390217 | LB1148 for Pulmonary Dysfunction Associated With COVID-19 Pneumonia | United States | Y | N | Shelved drug or NME | LB1148 | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04445246 | Inhaled Iloprost for Suspected COVID-19 Respiratory Failure | Qatar | N | N | Drug Repurposing | Iloprost | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04348461 | BAttLe Against COVID-19 Using MesenchYmal Stromal Cells | Spain | Y | N | Cell/Blood based | ND:MSC | Efficacy - Mortality | Severe Cases |
| NCT04365517 | The Effect of Sitagliptin Treatment in COVID-19 Positive Diabetic Patients | Italy | Y | N | Drug Repurposing | Sitagliptin | Efficacy - WHO scale | Mild/Moderate Cases, Other |
| NCT04364009 | Anakinra for COVID-19 Respiratory Symptoms | France | Y | N | Drug Repurposing | Anakinra | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04357860 | Clinical Trial of Sarilumab in Adults With COVID-19 | Spain | Y | N | Drug Repurposing | Sarilumab | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04355728 | Use of UC-MSCs for COVID-19 Patients | United States | Y | N | Cell/Blood based | ND:Stem cells | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04471519 | Whole-Virion Inactivated SARS-CoV-2 Vaccine (BBV152) for COVID-19 in Healthy Volunteers | India | Y | Y | Vaccine | BBV152 Vaccine | Safety | General population |
| NCT04429529 | Safety of TY027, a Treatment for COVID-19, in Humans | Singapore | Y | N | Shelved drug or NME | TY027 | Safety | Other |
| NCT04350086 | Use of Dexmedetomidine in Light to Moderate Sedation in the Patient in the Palliative Situation of a Sars-cov-2 / COVID-19 Infection | France | N | N | Drug Repurposing | Dexmedetomidine | Efficacy - Other efficacy | Severe Cases |
| NCT04361253 | Evaluation of SARS-CoV-2 (COVID-19) Antibody-containing Plasma thErapy | United States | Y | N | Cell/Blood based | Convalescent plasma | Efficacy - WHO scale | Severe Cases |
| NCT04452097 | Use of hUC-MSC Product (BX-U001) for the Treatment of COVID-19 With ARDS | United States | N | Y | Cell/Blood based | ND:Stem cells | Safety | Severe Cases |
| NCT04380935 | Effectiveness and Safety of Convalescent Plasma Therapy on COVID-19 Patients With Acute Respiratory Distress Syndrome | Indonesia | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| NCT04389450 | Double-Blind, Multicenter, Study to Evaluate the Efficacy of PLX PAD for the Treatment of COVID-19 | Israel | Y | Y | Shelved drug or NME | PLX-PAD | Efficacy - Other efficacy | Severe Cases |
| NCT04439071 | A Study to Evaluate Efficacy and Safety of PTC299 in Hospitalized Participants With Coronavirus (COVID-19) | United States | Y | N | Shelved drug or NME | PTC299 | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04483973 | SPI-1005 Treatment in Severe COVID-19 Patients | United States | Y | N | Drug Repurposing | Ebselen | Safety | Severe Cases |
| NCT04409834 | Prevention of Arteriovenous Thrombotic Events in Critically-Ill COVID-19 Patients Trial | United States | Y | Y | Drug Repurposing | Heparin, Enoxaparin, Clopidogrel | Efficacy - Mortality | Severe Cases |
| NCT04303507 | Chloroquine/ Hydroxychloroquine Prevention of Coronavirus Disease (COVID-19) in the Healthcare Setting | United Kingdom | Y | N | Drug Repurposing | Chloroquine, Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04484025 | SPI-1005 Treatment in Moderate COVID-19 Patients | United States | Y | N | Drug Repurposing | Ebselen | Safety | Mild/Moderate Cases |
| NCT04347226 | Anti-Interleukin-8 (Anti-IL-8) for Patients With COVID-19 | United States | Y | N | Shelved drug or NME | BMS-986253 | Efficacy - WHO scale | Other |
| NCT04379492 | A Study of Hydroxycholoroquine Compared to Placebo as Treatment for People With COVID-19 | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04369469 | Efficacy and Safety Study of IV Ravulizumab in Patients With COVID-19 Severe Pneumonia | United States | Y | N | Drug Repurposing | Ravulizumab | Efficacy - Mortality | Severe Cases |
| NCT04482712 | Effects of mTOR Inhibition With Sirolimus (RAPA) in Patients With COVID-19 to Moderate the Progression of ARDS | United States | Y | N | Drug Repurposing | Sirolimus | Efficacy - Mortality | Mild/Moderate Cases, Other |
| NCT04328480 | The ECLA PHRI COLCOVID Trial. Effects of Colchicine on Moderate/High-risk Hospitalized COVID-19 Patients. | Argentina | Y | N | Drug Repurposing | Colchicine | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04435522 | Maraviroc in Patients With Moderate and Severe COVID-19 | United States | N | N | Drug Repurposing | Maraviroc | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04357028 | Measles Vaccine in HCW | Egypt | Y | N | Drug Repurposing, Vaccine | Measles Vaccine | Efficacy - Infection rate (prevention) | General population |
| NCT04441918 | Tolerability,Safety,Pharmacokinetic Profile and Immunogenicity of a Recombinant Humanized Anti-SARS-CoV-2 Monoclonal Antibody (JS016) for Injection in Chinese Health Subjects | China | Y | N | Shelved drug or NME | JS016 | Safety | Other |
| NCT04429854 | Donated Antibodies Working Against nCoV | Belgium | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04483375 | Safety, Tolerability and Pharmacokinetics of SCTA01, an Anti-SARS-CoV-2 Monoclonal Antibody, in Healthy Chinese Subjects | China | Y | N | Shelved drug or NME | SCTA01 | Safety | Other |
| NCT04356508 | COVID-19: A Pilot Study of Adaptive Immunity and Anti-PD1 | Hong Kong | N | N | Drug Repurposing | Nivolumab | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04458948 | Open Label Non-comparative Trial of the Combination of Hydroxychloroquine and Azithromycin in the Treatment of Hospitalized Patients | United States | N | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04359290 | Ruxolitinib for Treatment of Covid-19 Induced Lung Injury ARDS | Germany | N | N | Drug Repurposing | Ruxolitinib | Efficacy - Mortality | Severe Cases |
| NCT04400929 | Using GM-CSF as a Host Directed Therapeutic Against COVID-19 | Singapore | Y | Y | Drug Repurposing | Sargramostim | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04346329 | Immune Monitoring of Prophylactic Effect of Hydroxychloroquine in Healthcare Providers Highly Exposed to COVID-19 | Colombia | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04335032 | EPA-FFA to Treat Hospitalised Patients With COVID-19 (SARS-CoV-2) | United Kingdom | N | N | Shelved drug or NME | Eicosapentaenoic acid | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04401150 | Lessening Organ Dysfunction With VITamin C - COVID-19 | Canada | Y | N | Drug Repurposing | Ascorbic acid | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04365153 | Canakinumab in Covid-19 Cardiac Injury (The Three C Study) | United States | Y | Y | Drug Repurposing | Canakinumab | Efficacy - WHO scale | Severe Cases |
| NCT04382846 | Novel Regimens in COVID-19 Treatment | Egypt | Y | Y | Drug Repurposing | Nitazoxanide, Ivermectin, Chloroquine, Azithromycin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04401527 | Treatment of Lung Injury From COVID-19 Infection With Intravenous Sodium Nitrite | United States | Y | N | Shelved drug or NME | Sodium Nitrite | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04421508 | A Study to Assess Pulsed Inhaled Nitric Oxide vs Placebo in Subjects With Mild or Moderate COVID-19 | United States | Y | N | Drug Repurposing | Nitric Oxide | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04453852 | Monovalent Recombinant COVID19 Vaccine | Australia | Y | N | Vaccine | Covax-19 vaccine | Safety | General population |
| NCT04405999 | Prevention of Infection and Incidence of COVID-19 in Medical Personnel Assisting Patients With New Coronavirus Disease | Russian Federation | Y | N | Drug Repurposing | Bromhexine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04365439 | Convalescent Plasma for COVID-19 | Italy | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Viral load | Severe Cases |
| NCT04472585 | Efficacy of Subcutaneous Ivermectin With or Without Zinc and Nigella Sativa in COVID-19 Patients | Pakistan | Y | Y | Drug Repurposing, Other | Ivermectin, Zinc, ND:Nigella sativa | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04324021 | Efficacy and Safety of Emapalumab and Anakinra in Reducing Hyperinflammation and Respiratory Distress in Patients With COVID-19 Infection. | Italy | Y | Y | Drug Repurposing | Emapalumab, Anakinra | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| ChiCTR2000 029939 |
A Single-blind, Randomized, Controlled Clinical Trial for Chloroquine Phosphate in the treatment of Novel Coronavirus Pneumonia 2019 (COVID-19) | China | Y | N | Drug Repurposing | Chloroquine | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029935 |
A Single-arm Clinical Trial for Chloroquine Phosphate in the treatment of Novel Coronavirus Pneumonia 2019 (COVID-19) | China | N | N | Drug Repurposing | Chloroquine | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029853 |
A randomized, open-label, controlled clinical trial for azvudine in the treatment of novel coronavirus pneumonia (COVID-19) | China | Y | N | Shelved drug or NME | Azvudine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029850 |
Effecacy and safty of convalescent plasma treatment for severe patients with novel coronavirus pneumonia (COVID-19): a prospective cohort study | China | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| ChiCTR2000 029806 |
Optimization of treatment and diagnosis plan for critically ill patients | China | Y | Y | Drug Repurposing, Shelved drug or NME | Thymosin, Camrelizumab | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| ChiCTR2000 029776 |
A randomized, open-label, blank-controlled, multicenter trial for Polyinosinic-Polycytidylic Acid Injection in the treatment of novel coronavirus pneumonia (COVID-19) | China | N | N | Shelved drug or NME | Polyinosinic-Polycytidylic Acid | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029765 |
A multicenter, randomized controlled trial for the efficacy and safety of tocilizumab in the treatment of new coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029742 |
A randomized, parallel controlled trial for the efficacy and safety of Sodium Aescinate Injection in the treatment of patients with pneumonia (COVID-19) | China | Y | N | Shelved drug or NME | Sodium Aescinate | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029741 |
Efficacy of Chloroquine and Lopinavir/ Ritonavir in mild/general novel coronavirus (CoVID-19) infections: a prospective, open-label, multicenter randomized controlled clinical study | China | Y | N | Drug Repurposing | Chloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| ChiCTR2000 029656 |
A randomized, open-label study to evaluate the efficacy and safety of low-dose corticosteroids in hospitalized patients with novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Methylprednisolone | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029636 |
Efficacy and safety of aerosol inhalation of vMIP in the treatment of novel coronavirus pneumonia (COVID-19): a single arm clinical trial | China | N | N | Shelved drug or NME | vMIP | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029609 |
A prospective, open-label, multiple-center study for the efficacy of chloroquine phosphate in hospitalized patients with novel coronavirus pneumonia (COVID-19) | China | Y | Y | Drug Repurposing | Chloroquine, Lopinavir/ritonavir | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029606 |
Clinical Study for Human Menstrual Blood-derived Stem Cells in the Treatment of Acute Novel Coronavirus Pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:MSC | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029600 |
Clinical study on safety and efficacy of Favipiravir in the treatment of novel coronavirus pneumonia (COVID-19) | China | Y | Y | Drug Repurposing | Favipiravir, Lopinavir/ritonavir, Interferon alpha | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029569 |
Safety and efficacy of umbilical cord blood mononuclear cells conditioned medium in the treatment of severe and critically novel coronavirus pneumonia (COVID-19): a randomized controlled trial | China | Y | N | Shelved drug or NME | ND:MSC | Efficacy - Other efficacy | Severe Cases |
| ChiCTR2000 029559 |
Therapeutic effect of hydroxychloroquine on novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029544 |
A randomized controlled trial for the efficacy and safety of Baloxavir Marboxil, Favipiravir tablets in novel coronavirus pneumonia (COVID-19) patients who are still positive on virus detection under the current antiviral therapy | China | Y | Y | Drug Repurposing | Baloxavir Marboxil, Favipiravir | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029542 |
A prospective cohort study for the efficacy and safety of chloroquine in hospitalized patients with novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Chloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029541 |
A randomised, open, controlled trial for darunavir/cobicistat or Lopinavir/ritonavir combined with thymosin a1 in the treatment of novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Darunavir, Cobicistat, Lopinavir/ritonavir, Thymosin alpha | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029539 |
A randomized, open-label study to evaluate the efficacy and safety of Lopinavir-Ritonavir in patients with mild novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Lopinavir/ritonavir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| ChiCTR2000 029468 |
The early use of lopinavir/litonavir (LPV/r) and emtritabine (FTC)/ Tenofovir alafenamide Fumarate tablets (TAF) regimen in the treatment of the novel coronavirus pneumonia (COVID-19): a real-world study | China | Y | N | Drug Repurposing | Lopinavir/ritonavir, Emtricitabine, Tenofovir alafenamide | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030179 |
Experimental study of novel coronavirus pneumonia rehabilitation plasma therapy severe novel coronavirus pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030173 |
Key techniques of umbilical cord mesenchymal stem cells for the treatment of novel coronavirus pneumonia (COVID-19) and clinical application demonstration | China | Y | N | Cell/Blood based | ND:MSC | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030170 |
Study for safety and efficacy of Jakotinib hydrochloride tablets in the treatment severe and acute exacerbation patients of novel coronavirus pneumonia (COVID-19) | China | N | N | Shelved drug or NME | Jaktinib | Safety | Severe Cases |
| ChiCTR2000 030167 |
Clinical Trial for Recombinant Human Interleukin-2 in the Treatment of Novel Coronavirus Pneumonia (COVID-19) | China | Y | N | Drug Repurposing | recombinant human IL-2 | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030138 |
Clinical Trial for Human Mesenchymal Stem Cells in the Treatment of Severe Novel Coronavirus Pneumonia (COVID-19) | China | Y | N | Shelved drug or NME | ND:MSC | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030116 |
Safety and effectiveness of human umbilical cord mesenchymal stem cells in the treatment of acute respiratory distress syndrome of severe novel coronavirus pneumonia (COVID-19) | China | N | N | Cell/Blood based | ND:MSC | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| ChiCTR2000 030113 |
Clinical study for safety and efficacy of Favipiravir in the treatment of novel coronavirus pneumonia (COVID-19) with poorly responsive ritonavir/ritonavir | China | Y | N | Drug Repurposing | Favipiravir | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030089 |
A randomized, open-label, controlled trial for the efficacy and safety of Adalimumab Injection in the treatment of patients with severe novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Adalimumab | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| ChiCTR2000 030088 |
Umbilical cord Wharton's Jelly derived mesenchymal stem cells in the treatment of severe novel coronavirus pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:MSC | Efficacy - Viral load | Severe Cases |
| ChiCTR2000 030058 |
A multicenter, randomized, double-blind, controlled clinical trial for leflunomide in the treatment of novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Leflunomide | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030054 |
An open randomized controlled trial for Chloroquine phosphate and Hydroxychloroquine sulfate in the treatment of mild and common novel coronavirus pneumonia (COVID-19) | China | Y | Y | Drug Repurposing | Chloroquine, Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030046 |
A single arm trial to evaluate the efficacy and safety of anti-2019-nCoV inactivated convalescent plasma in the treatment of novel coronavirus pneumonia patient (COVID-19) | China | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030041 |
A single-arm, single-center clinical trial for Azivudine tablets in the treatment of adult novel coronavirus pneumonia (COVID-19) | China | N | N | Shelved drug or NME | Azvudine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030039 |
Clinical study for infusing convalescent plasma to treat patients with new coronavirus pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030029 |
A multi-center study on the efficacy and safety of suramin sodium in adult patients with novel coronavirus pneumonia (COVID-19) | China | N | N | Drug Repurposing | Suramin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030020 |
The clinical application and basic research related to mesenchymal stem cells to treat novel coronavirus pneumonia (COVID-19) | China | N | N | Shelved drug or NME | ND:MSC | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030016 |
Efficacy evaluation of inhalation of mycobacterium vaccae injection for treating Novel coronavirus pneumonia (COVID-19) and the mechanism study of prevention and treatment of respiratory virus infection | China | Y | N | Shelved drug or NME | ND:Mycobacterium | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030010 |
A randomized, double-blind, parallel-controlled, trial to evaluate the efficacy and safety of anti-SARS-CoV-2 virus inactivated plasma in the treatment of severe novel coronavirus pneumonia patients (COVID-19) | China | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030007 |
Multicenter randomized controlled trial for rhG-CSF in the treatment of novel coronavirus pneumonia (COVID-19) | China | Y | N | Cell/Blood based | G-CSF | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030002 |
Clinical study for novel NLRP Inflammasome inhibitor (Tranilast) in the treatment of novel coronavirus (COVID-19) | China | Y | N | Drug Repurposing | Tranilast | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030001 |
The efficacy and safety of Triazavirin for 2019 novel coronary pneumonia (COVID-19): a multicenter, randomized, double blinded, placebo-controlled trial | China | Y | N | Shelved drug or NME | Triazavirin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029996 |
A randomized, open-label, controlled trial for the efficacy and safety of Farpiravir Tablets in the treatment of patients with novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Favipiravir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029990 |
Clinical trials of mesenchymal stem cells for the treatment of pneumonitis caused by novel coronavirus (COVID-19) | China | Y | N | Shelved drug or NME | ND:MSC | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029989 |
A randomized controlled Trial for therapeutic efficacy of Recombinant Human Interferon alpha 1b Eye Drops in the treatment of elderly with novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Interferon alpha 1b | Efficacy - Oxygen parameters | Other |
| ChiCTR2000 029988 |
Clinical Study of Chloroquine Phosphate in the Treatment of Severe Novel Coronavirus Pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Chloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029851 |
A multicenter, randomized controlled trial for the efficacy and safety of Alpha lipoic acid (iv) in the treatment of patients of severe novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Alpha Lipoic Acid | Efficacy - Other efficacy | Severe Cases |
| ChiCTR2000 029803 |
A prospective, randomized, open-label, controlled clinical study to evaluate the preventive effect of hydroxychloroquine on close contacts after exposure to the Novel Coronavirus Pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Hydroxychloroquine, Umifenovir | Efficacy - Infection rate (prevention) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029740 |
Efficacy of therapeutic effects of hydroxycholoroquine in novel coronavirus pneumonia (COVID-19) patients(randomized open-label control clinical trial) | China | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030442 |
The therapeutic efficacy of combination of Tocilizumab, IVIG and CRRT in sever patients with novel coronavirus pneumonia (COVID-19) | China | N | N | Drug Repurposing, Cell/Blood based | Tocilizumab | Efficacy - Other efficacy | Severe Cases |
| ChiCTR2000 030424 |
A single-center, single-arm clinical trial for azvudine in the treatment of novel coronavirus pneumonia (COVID-19) | China | N | N | Shelved drug or NME | Azvudine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030398 |
A randomized, double-blind, placebo-controlled trial for evaluation of the efficacy and safety of bismuth potassium citrate capsules in the treatment of patients with novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Bismuth potassium citrate | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030265 |
Clinical research program of continuous renal replacement therapy with adsorption filter for the treatment of the novel coronavirus pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:Continuous renal replacement therapy | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030261 |
A study for the key technology of mesenchymal stem cells exosomes atomization in the treatment of novel coronavirus pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:MSC | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030196 |
A multicenter, single arm, open label trial for the efficacy and safety of CMAB806 in the treatment of cytokine release syndrome of novel coronavirus pneumonia (COVID-19) | China | N | N | Drug Repurposing | Tocilizumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030013 |
A study on the efficacy and safety of recombinant human interferon alpha 1b spray in preventing novel coronavirus (COVID-19) infection in highly exposed medical staffs. | China | Y | N | Drug Repurposing | Interferon alpha 1b | Safety | Exposed individuals (family members, HCP) |
| ChiCTR2000 029975 |
Single arm study for exploration of chloroquine phosphate aerosol inhalation in the treatment of novel coronavirus pneumonia (COVID-19) | China | N | N | Drug Repurposing | Chloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029899 |
Evaluation the Efficacy and Safety of Hydroxychloroquine Sulfate in Comparison with Phosphate Chloroquine in Mild and Commen Patients with Novel Coronavirus Pneumonia (COVID-19): a Randomized, Open-label, Parallel, Controlled Trial | China | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| ChiCTR2000 029898 |
Evaluation the Efficacy and Safety of Hydroxychloroquine Sulfate in Comparison with Phosphate Chloroquine in Severe Patients with Novel Coronavirus Pneumonia (COVID-19): a Randomized, Open-Label, Parallel, Controlled Trial | China | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| ChiCTR2000 029762 |
A randomized, open-label, controlled trial for the efficacy and safety of hydroxychloroquine sulfate tablets in the treatment of patients with severe novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Severe Cases |
| ChiCTR2000 029761 |
Multicenter, randomized, open clinical study of Hydroxychloroquine Sulfate tablets in the treatment of common patients with novel coronavirus pneumonia (COVID-19) | China | Y | Y | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases |
| ChiCTR2000 029760 |
A randomized controlled study to evaluate the efficacy and safety of hydroxychloroquine for mild and moderate COVID-19 Infectious diseases | China | Y | N | Drug Repurposing | Hydroxychloroquine, Lopinavir/ritonavir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| ChiCTR2000 029387 |
Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha and ribavirin plus lopinavir/ritonavir plus interferon-alphain in patients with mild to moderate novel coronavirus pneumonia | China | Y | Y | Drug Repurposing | Ribavirin, Interferon alpha, Lopinavir/ritonavir | Efficacy - Viral load | Mild/Moderate Cases |
| ChiCTR2000 029386 |
A study on treatment strategies of novel coronavirus pnueumonia patients | China | Y | N | Drug Repurposing | Methylprednisolone | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030627 |
Study on the application of convalescent plasma therapy in severe COVID-19 | China | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Viral load | Severe Cases |
| ChiCTR2000 030535 |
Multi-Center Clinical Study on the Treatment of Patients with Novel Coronavirus Pneumonia (COVID-19) by Ebastine | China | Y | N | Drug Repurposing | Ebastine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030487 |
A single-center, single-arm clinical trial for azvudine in the treatment of novel coronavirus pneumonia (COVID-19) | China | N | N | Shelved drug or NME | Azvudine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030484 |
HUMSCs and Exosomes Treating Patients with Lung Injury following Novel Coronavirus Pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:MSC | Efficacy - Oxygen parameters | Other |
| ChiCTR2000 030472 |
An open and controlled clinical study to evaluate the efficacy and safety of Ganovo combined with ritonavir in the treatment of novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing, Shelved drug or NME | Danoprevir, Ritonavir | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030471 |
Efficacy and safety of lipoic acid injection in reducing the risk of progression in common patients with novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Alpha Lipoic Acid | Efficacy - Other efficacy | Mild/Moderate Cases |
| ChiCTR2000 030312 |
A single-center, open-label and single arm trial to evaluate the efficacy and safety of anti-SARS-CoV-2 inactivated convalescent plasma in the treatment of novel coronavirus pneumonia (COVID-19) patient | China | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029757 |
Convalescent plasma for the treatment of severe and critical novel coronavirus pneumonia (COVID-19): a prospective randomized controlled trial | China | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| ChiCTR2000 029580 |
Severe novel coronavirus pneumonia (COVID-19) patients treated with ruxolitinib in combination with mesenchymal stem cells: a prospective, single blind, randomized controlled clinical trial | China | Y | N | Drug Repurposing, Cell/Blood based | Ruxolitinib, ND:MSC | Safety | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029308 |
A randomized, controlled open-label trial to evaluate the efficacy and safety of lopinavir-ritonavir in hospitalized patients with 2019-nCoV infection | China | Y | N | Drug Repurposing | Lopinavir/ritonavir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030892 |
Efficacy and Safety of Pirfenidone in the Treatment of Severe Post-Novel Coronavirus Pneumonia (COVID-19)) Fibrosis:a prospective exploratory experimental medical study | China | Y | N | Drug Repurposing | Pirfenidone | Efficacy - Other efficacy | Severe Cases |
| ChiCTR2000 030853 |
Evaluation of the protective effect of dexmedetomidine on patients with severe new coronavirus pneumonia | China | N | N | Drug Repurposing | Dexmedetomidine | Efficacy - Other efficacy | Severe Cases |
| ChiCTR2000 030841 |
Treatment of Acute Severe COVID-19 With Immunoglobulin From Cured COVID-19 Patients | China | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| ChiCTR2000 030835 |
Clinical study on the efficacy of Mesenchymal stem cells (MSC) in the treatment of severe novel coronavirus pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:MSC | Efficacy - Other efficacy | Severe Cases |
| ChiCTR2000 030779 |
A randomized, open-label, controlled trial for the efficacy and safety of Ulinastatin Injection in the treatment of patients with severe novel coronavirus pneumonia (COVID-19) | China | Y | N | Shelved drug or NME | Ulinastatin | Efficacy - Oxygen parameters | Severe Cases |
| ChiCTR2000 030718 |
Clinical Study of Chloroquine Phosphate in the Treatment of 2019-nCoV Infection | China | Y | N | Drug Repurposing | Chloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030703 |
A randomized, blinded, controlled, multicenter clinical trial to evaluate the efficacy and safety of Ixekizumab combined with conventional antiviral drugs in patients with novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Ixekizumab | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030702 |
Convalescent plasma for the treatment of common COVID-19: a prospective randomized controlled trial | China | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| ChiCTR2000 030701 |
A randomized, parallel controlled open-label trial to evaluate the efficacy and safety of Prolongin (Enoxaparin Sodium Injection) in adult hospitalized patients with novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Enoxaparin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030700 |
An evaluative clinical study: efficacy and safety of of Prolongin (Enoxaparin Sodium Injection) in treatment of hospitalized adult patients with common novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Enoxaparin | Efficacy - Viral load | Mild/Moderate Cases |
| ChiCTR2000 030580 |
Tozumab combined with adamumab(Qletli) in the treatment of severe and critically ill patients with novel coronavirus pneumonia (COVID-19):A prospective, single-center, randomized, parallel controlled trial | China | Y | N | Drug Repurposing | Adalimumab, Tocilizumab | Efficacy - Other efficacy | Severe Cases |
| ChiCTR2000 030509 |
Clinical Study of NK Cells in the Treatment of Novel Coronavirus Pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:NK cells | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030417 |
Efficacy and safety of chloroquine phosphate inhalation combined with standard therapy in the treatment of novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Chloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030381 |
A randomized, open-label, controlled and single-center trial to evaluate the efficacy and safety of anti-SARS-CoV-2 inactivated convalescent plasma in the treatment of novel coronavirus pneumonia (COVID-19) patient | China | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030329 |
Clinical trial for umbilical cord blood CIK and NK cells in the treatment of mild and general patients infected with novel coronavirus pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:Umbilical cord NK/CIK cells | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030187 |
Efficacy and safety of Lopinavir and Ritonavir in the treatment of novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Lopinavir/ritonavir | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030135 |
A randomized controlled trial for high-dose Vitamin C in the treatment of severe and critical novel coronavirus pneumonia (COVID-19) patients | China | Y | N | Drug Repurposing | Ascorbic acid | Efficacy - Mortality | Severe Cases |
| ChiCTR2000 030082 |
Randomized controlled trial for the efficacy of dihydroartemisinine piperaquine in the treatment of mild/common novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Dihydroartemisinin/piperaquine | Efficacy - Viral load | Mild/Moderate Cases |
| ChiCTR2000 030031 |
A randomized, double-blind, parallel, controlled trial for comparison of phosphoric chloroquine combined with standard therapy and standard therapy in mild/common patients with novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Chloroquine | Efficacy - Viral load | Mild/Moderate Cases |
| ChiCTR2000 029984 |
Study on the effect of human placenta biological preparation on the defense of immune function against novel coronavirus pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:Human placenta extract | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029895 |
Single arm study for the efficacy and safety of GD31 in patients with novel coronavirus pneumonia (COVID-19) | China | N | N | Shelved drug or NME | GD31 | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029837 |
A randomized, double-blind, parallel, controlled trial for comparison of phosphoric chloroquine combined with standard therapy and standard therapy in mild/common patients with novel coronavirus pneumon (COVID-19)ia | China | Y | N | Drug Repurposing | Chloroquine | Efficacy - Viral load | Mild/Moderate Cases |
| ChiCTR2000 029826 |
A randomized, double-blind, parallel, controlled trial for comparison of phosphoric chloroquine combined with standard therapy and standard therapy in serious/critically ill patients with novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Chloroquine | Efficacy - Mortality | Severe Cases |
| ChiCTR2000 029817 |
Clinical Study of Cord Blood NK Cells Combined with Cord Blood Mesenchymal Stem Cells in the Treatment of Acute Novel Coronavirus Pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:MSC, ND:NK cells | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| ChiCTR2000 029812 |
Clinical Study for Umbilical Cord Blood Mononuclear Cells in the Treatment of Acute Novel Coronavirus Pneumonia (NCP) | China | Y | N | Cell/Blood based | ND:Cord blood MNC | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| ChiCTR2000 031139 |
Safety and Effectiveness of Human embryonic stem cell-derived M cells (CAStem) for Pulmonary Fibrosis Correlated with novel coronavirus pneumonia(COVID-19) | China | N | N | Cell/Blood based | ND:MSC | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 031138 |
Prospective exploratory clinical research on the efficacy and safety of pirfenidone capsules in the treatment of severe novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Pirfenidone | Efficacy - Other efficacy | Severe Cases |
| ChiCTR2000 030987 |
Clinical Trial of Favipiravir Tablets Combine With Chloroquine Phosphate in the Treatment of novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Chloroquine, Favipiravir | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030944 |
An open, multi-center, control, exploratory clinical study of human NK cells and UC-MSCs transplantation for severe novel coronavirus pneumonia | China | Y | N | Cell/Blood based | ND:NK cells, ND:MSC | Efficacy - Other efficacy | Severe Cases |
| ChiCTR2000 030939 |
Preliminary evaluation of the safety and efficacy of oral LL-37 antiviral peptide (CAS001) in the treatment of novel coronavirus pneumonia | China | N | N | Shelved drug or NME | CSA0001 | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030929 |
A randomized, double-blind, parallel-controlled trial to evaluate the efficacy and safety of anti-SARS-CoV-2 virus inactivated plasma in the treatment of severe novel coronavirus pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| ChiCTR2000 030922 |
Prospective, open-label, controlled, multicenter cohort study of long-acting interferon plus ribavirin in patients with COVID-19 infection | China | Y | N | Drug Repurposing | Interferon alpha 2a | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030906 |
A single-center, open and dose-escalation phase I clinical trial for recombinant novel coronavirus vaccine (adenoviral vector) in healthy adults aged between 18 and 60 years | China | Y | N | Vaccine | Adenoviral vaccine | Safety | General population |
| ChiCTR2000 030539 |
Study for the effect of 3% hydrogen peroxide gargle on the Intraoral novel coronavirus of the patients with novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Hydrogen peroxide | Efficacy - Viral load | Mild/Moderate Cases |
| ChiCTR2000 030254 |
the Efficacy and Safety of Favipiravir for novel coronavirus–infected pneumonia: A multicenter, randomized, open, positive, parallel-controlled clinical study | China | Y | N | Drug Repurposing | Favipiravir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030224 |
Clinical study of mesenchymal stem cells in treating severe novel coronavirus pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:MSC | Efficacy - Oxygen parameters | Severe Cases |
| ChiCTR2000 029868 |
Hydroxychloroquine treating novel coronavirus pneumonia (COVID-19): a randomized controlled, open label, multicenter trial | China | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029496 |
A randomized, open label, parallel controlled trial for evaluating the efficacy of recombinant cytokine gene-derived protein injection on clearing novel coronavirus in patients with novel coronavirus pneumonia (COVID-19) | China | Y | N | Shelved drug or NME | Novaferon | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 031204 |
A multicenter, single-blind, randomized controlled clinical trial for chloroquine phosphate in the treatment of 2019 novel coronavirus-infected pneumonia | China | Y | N | Drug Repurposing | Chloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 031196 |
Clinical efficacy of novel coronavirus infection treated by combination of interferon regimen and Ritonavir Tablets | China | Y | N | Drug Repurposing | Lopinavir/ritonavir, Interferon | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030028 |
Clinical comparative study of PD-1 mAb in the treatment of severe and critical patients with novel coronavirus disease (COVID-19) | China | Y | N | Drug Repurposing | Anti-PD1 mab (unspecified) | Efficacy - Other efficacy | Severe Cases |
| ChiCTR2000 031630 |
Clinical study for Celebrex in the treatment of novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Celecoxib | Other | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 031501 |
The efficacy of convalescent plasma in patients with critical novel coronavirus pneumonia (COVID-19): a pragmatic, prospective cohort study | China | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| ChiCTR2000 031494 |
Clinical study for stem cells in the treatment of severe novel coronavirus pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:MSC | Efficacy - Other efficacy | Severe Cases |
| ChiCTR2000 031454 |
Clinical study for prevention and treatment of digestive tract lesions caused by novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Rabeprazole | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 031430 |
Evaluation of the safety and efficacy for human umbilical cord mesenchymal stem cells in COVID-19 induced pulmonary fibrosis | China | Y | N | Cell/Blood based | ND:MSC | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ACTRN12620 000454976 |
High-dose intravenous zinc (HDIVZn) as adjunctive therapy in COVID-19 positive critically ill patients: A pilot randomized controlled trial | Australia | Y | N | Drug Repurposing | Zinc chloride | Efficacy - Oxygen parameters | Severe Cases |
| ChiCTR2000 031781 |
A randomized, double-blinded, placebo-controlled phase II clinical trial for Recombinant Novel Coronavirus (2019-nCOV) Vaccine (Adenovirus Vector) in healthy adults aged above 18 years | China | Y | N | Vaccine | Adenoviral vaccine | Efficacy - Other efficacy | General population |
| ChiCTR2000 031735 |
Clinical study for natural killer (NK) cells from umbilical cord blood in the treatment of viral pneumonia include novel coronavirus pneumonia (COVID-19) | China | Y | N | Cell/Blood based | ND:NK cells | Safety | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030750 |
A clinical study for effectiveness and safety evaluation for recombinant chimeric COVID-19 epitope DC vaccine in the treatment of novel coronavirus pneumonia | China | Y | N | Vaccine | Chimeric COVID-19 DC vaccine | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 030328 |
Clinical application of inhaled acetylcysteine solution in the treatment of novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | N-acetylcysteine | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| ACTRN12620 000473965 |
Reducing acute severe respiratory events in health care workers during the Covid-19 pandemic with OM85 | Australia | Y | N | Drug Repurposing | OM-85 | Efficacy - Other efficacy | Exposed individuals (family members, HCP) |
| ACTRN12620 000478910 |
Cord Blood Therapy to prevent progression of COVID-19 related pneumonia | Australia | Y | N | Cell/Blood based | ND:Stem cells | Safety | Mild/Moderate Cases |
| ACTRN12620 000480987 |
Stress-reduction Using Probiotics to Promote Ongoing Resilience Throughout COVID-19 for Healthcare Workers: A randomised placebo-controlled trial | New Zealand | Y | N | Drug Repurposing | Lactobacillus rhamnosus HN001 | Other | Exposed individuals (family members, HCP) |
| ChiCTR2000 030300 |
Umbilical cord mesenchymal stem cells for the treatment of patients at high risk of novel coronavirus pneumonia (COVID-19): a single-center, prospective, open clinical study | China | N | N | Cell/Blood based | ND:MSC | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202003 10046736N1 |
Comparison of The Therapeutic Effect of Convalescent Plasma and Plasma-derived Immunoglobulin-enriched solution on COVID-19 Patients: A Clinical Trial Study | Iran | Y | Y | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Severe Cases |
| IRCT201002 28003449N30 |
Evaluating efficacy and safety of hydroxychloroquine + lopinavir or atazanavir/ritonavir combination in patients with COVID-19 | Iran | N | N | Drug Repurposing | Hydroxychloroquine, Atazanavir/ritonavir, Lopinavir/ritonavir | Efficacy - Other efficacy | Mild/Moderate Cases |
| IRCT201002 28003449N28 |
Evaluating efficacy and safety of interferone Ăź-1a in the treatment COVID-19 infection | Iran | Y | N | Drug Repurposing | Interferon beta-1a | Efficacy - Other efficacy | Mild/Moderate Cases |
| IRCT201002 28003449N27 |
Evaluating efficacy and safety of interferone Ăź-1b (IFN Ăź-1b) in the treatment of COVID-19 | Iran | Y | N | Drug Repurposing | Interferon beta-1b | Efficacy - Other efficacy | Mild/Moderate Cases |
| IRCT201512 27025726N12 |
Evaluting the therapeutic and adverse effects of Interferon beta 1-a subcutaneous administration in patients with novel Coronavirus (COVID-19) | Iran | N | N | Drug Repurposing | Interferon beta-1a | Efficacy - Other efficacy | Severe Cases |
| IRCT201701 17032004N3 |
evaluation the effect of vitamin A on respiratory signs and hospitalization in patients with COVID-19 | Iran | Y | N | Drug Repurposing | Vitamin A | Efficacy - Other efficacy | Mild/Moderate Cases |
| IRCT201002 28003449N29 |
Evaluating efficacy and safety of sofosbuvir/ ledipasvir in treatment of COVID-19 | Iran | Y | N | Drug Repurposing | Ledipasvir/sofosbuvir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| IRCT202003 17046797N1 |
Effect of Camostate mesylate on clinical improvement and outcome of Coronavirus (COVID-19)-induced pneumonia | Iran | Y | N | Drug Repurposing | Camostat | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201606 25028622N1 |
The effect of NOSCOVID on pulmonary & other clinical manifestations of COVID-19 patients | Iran | Y | N | Drug Repurposing | Noscapine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201208 26010664N6 |
Effect of hydroxychloroquine on prevention of covid-19 virus infection among treatment staff in Arash hospital-A double-blind clinical trial | Iran | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| IRCT201405 28017891N8 |
Evaluation of the efficacy and safety of cord-derived mesenchymal stem cell transplantation in the treatment of COVID-19 | Iran | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Mortality | Severe Cases |
| IRCT202003 21046828N1 |
The effect of genetical nanocomposit drug with cell imunity level on human coronavirus (COVID-19) | Iran | Y | Y | Shelved drug or NME | NOS | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT201907 27044343N1 |
The effect of febuxostat against lung injury induced by COVID-19 virus in infected patients: A clinical trial study | Iran | Y | N | Drug Repurposing | Febuxostat | Efficacy - Other efficacy | Mild/Moderate Cases |
| IRCT201509 14024017N1 |
the efficacy of Interferon beta 1 a (ReciGen) in treatment of patients with Covid-19 in Sina Hospital Emergency Department | Iran | Y | N | Drug Repurposing | Interferon beta-1a | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT201409 11019125N6 |
Study the effect of intravenous injection of dental pulp mesenchymal stem cells in treatment of patients with COVID-19 pneumonia | Iran | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201512 27025726N13 |
Evaluation the Efficacy and Safety of Tocilizumab in Patients with Novel Coronavirus (COVID-19) | Iran | N | N | Drug Repurposing | Tocilizumab, Hydroxychloroquine, Oseltamivir, Lopinavir/Ritonavir, Interferon beta-1a | Efficacy - Other efficacy | Severe Cases |
| IRCT201603 10026998N10 |
Study of the metformin effect on the survival and recovery rate of COVID-19 patients | Iran | Y | N | Drug Repurposing | Metformin | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| IRCT202003 24046850N5 |
Study of the effect of Vitamin C on duration of hospital stay in patients with Covid-19 | Iran | Y | N | Drug Repurposing | Ascorbic acid | Efficacy - Other efficacy | Severe Cases |
| IRCT202003 25046860N1 |
Evaluation of Convalescent Plasma as a Potential Therapy for COVID-19 infected patients | Iran | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| IRCT202003 17046797N2 |
Effect of Fingolimod for treatment of COVID-19-induced cytokine storm | Iran | Y | N | Drug Repurposing | Fingolimod | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| IRCT202003 25046860N2 |
Mesenchymal stem cell utilization in reducing complications and enhancing pneumonia healing in patients infected with 2019-nCoV (phase I clinical trial) | Iran | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201909 17044805N2 |
Effects of High-dose Vitamin C on Treatment, Clinical Symptoms and Laboratory Signs of Iranian COVID-19 Patients: A Double Blind Randomized Clinical Trial | Iran | Y | N | Drug Repurposing | Ascorbic acid | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201202 25009124N4 |
Efficacy of different methods of administeration of combination regiemn including dexamethasone, IV-IG and Interferone beta for treatment of patients with severe COVID-19: a randomized controlled trial | Iran | Y | Y | Drug Repurposing | Dexamethasone, Interferon beta, ND:Immunoglobulin | Efficacy - Oxygen parameters | Severe Cases |
| IRCT201808 02040678N4 |
Evaluation of the effects of Losartan in patients with corona virus disease 2019 | Iran | Y | N | Drug Repurposing | Losartan | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| IRCT201507 04023055N2 |
To evaluate the effectiveness of hemoperfusion in patients with severe coronavirus disease 2019 (COVID-19) | Iran | N | N | Cell/Blood based | ND:Procedure | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| IRCT202003 19046819N1 |
Impact of vitamin B, A, D, E, C supplementation on improvement and mortality rate in patients with COVID-19 admitted in intensive care unit | Iran | Y | N | Drug Repurposing, Other | Ascorbic acid, ND:Vitamin supplementation | Efficacy - Other efficacy | Severe Cases |
| JPRN-Japic CTI-205238 |
Multicenter, Adaptive, Randomized, Placebo-Controlled, Comparative Study to Evaluate the Efficacy and Safety of Favipiravir in Patients with COVID-19 Non-Severe Pneumonia | Japan | Y | N | Drug Repurposing | Favipiravir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| ACTRN12620 000417987 |
Multi-Site, Randomized, Open-Label, Parallel-Group, Placebo-Controlled Study to Assess the Chemoprophylactic Efficacy of Chloroquine Against SARS-CoV-2/COVID-19 in Healthcare Workers at High-Risk of Exposure | Australia | Y | N | Drug Repurposing | Chloroquine | Efficacy - Other efficacy | Exposed individuals (family members, HCP) |
| ACTRN12620 000457943 |
A Randomised, Double Blind, Placebo-Controlled Trial of the Efficacy of Hydroxychloroquine for the Community-Based Treatment of Adults With Diagnosed COVID-19 | New Zealand | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 032400 |
the efficacy and safety of high dose intravenous vitamin C in the treatment of novel coronavirus pneumonia (COVID-19): a prospective, randomize, controlled trial | China | Y | N | Drug Repurposing | Ascorbic acid | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 032314 |
A randomized clinical trial for the efficacy and safety of Aliskiren and Nifedipine in novel coronavirus pneumonia (COVID-19) patients with hypertension | China | Y | Y | Drug Repurposing | Aliskiren, Nifedipine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases, Other |
| ChiCTR2000 032242 |
A multicenter, randomized, open, controlled trial for the efficacy and safety of oral kolimycin in the treatment of patients with new coronavirus pneumonia (CoVID-19) | China | Y | N | Drug Repurposing | Colistin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ACTRN12620 000517976 |
A randomised controlled trial of Nebulised Heparin in critically ill mechanically ventilated patients with COVID-19 to assess the effect on the duration of mechanical ventilation. | Australia | Y | N | Drug Repurposing | Heparin | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| ChiCTR2000 032487 |
Study for using sulfate in the prevention and control of novel coronavirus pneumonia (COVID-19) in high and low prevalence communities | China | Y | N | Drug Repurposing | Hydroxychloroquine | Other | General population |
| ChiCTR2000 032459 |
Evaluation of the safety and immunogenicity of inactivated novel coronavirus (2019-CoV) vaccine (Vero cells) in healthy population aged 3 years and above: a randomized, double-blind, placebo parallel-controlled phase I/II clinical trial | China | Y | N | Vaccine | ND:Vaccine | Safety | General population |
| ChiCTR2000 032135 |
Efficacy and Safety of ulinastatin in the Treatment of novel coronavirus pneumonia (COVID-19) | China | Y | Y | Shelved drug or NME | Ulinastatin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 031174 |
Effectiveness and safety of hydroxychloroquine sulfate in the preventive treatment of novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Hydroxychloroquine | Other | General population |
| ChiCTR2000 032769 |
A randomized,double blinded, parallel controlled clinical trial for azvudine in the treatment of novel coronavirus pneumonia (COVID-19) | China | Y | N | Shelved drug or NME | Azvudine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 032717 |
Efficacy and safety of high-dose vitamin C combined with traditional Chinese medicine in the treatment of moderate and severe novel coronavirus pneumonia (COVID-19) | China | Y | Y | Drug Repurposing, TCM | Ascorbic acid, ND:TCM | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 032716 |
High dose intravenous vitamin C might be used as an important rescue therapy for aggravation of severe and critical novel coronavirus pneumonia (COVID-19) patients | China | N | N | Drug Repurposing | Ascorbic acid | Efficacy - Other efficacy | Severe Cases |
| ChiCTR2000 033003 |
A safety and immune response study of novel coronavirus pneumonia (COVID-19) s-vax loaded autologous dendritic cells | China | N | N | Cell/Blood based | ND:Vaccine | Safety | General population |
| ChiCTR2000 032915 |
A randomized controlled trial for the efficacy and safety of artemisinin-pipequine tablets in the treatment of the mild and common type novel coronavirus pneumonia (COVID-19) patients whose nCoV Nucleic acid did not turn negative after treated by hydroxychloroquine and Abidor | China | Y | N | Drug Repurposing | Artemisinin-piperaquine | Safety | Mild/Moderate Cases |
| ChiCTR2000 030262 |
Clinical study of novel coronavirus (SARS-Cov-2) infection treated with aerosol inhalation (IFN-? plus anti-inflammatory factor TFF2) | China | Y | N | Shelved drug or NME | TFF2 | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 029638 |
A Multicenter, Randomized, Controlled trial for Recombinant Super-Compound Interferon (rSIFN-co) in the Treatment of Novel Coronavirus (2019-nCoV) Pneumonia | China | Y | N | Shelved drug or NME | rSIFN-co | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202003 14046764N1 |
The evaluation of pirfenidone effects on prevention and treatment of pulmonary fibrosis and acute respiratory distress syndrome caused by COVID-19 | Iran | Y | N | Drug Repurposing | Pirfenidone | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT201503 03021315N17 |
Evaluation of the safety and efficacy of tocilizumab (AryoGen ?Pharmed Co., Iran) in patients with severe COVID-19? | Iran | N | N | Drug Repurposing | Tocilizumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202003 12046749N1 |
Evaluation the efficacy of Tumor Necrosis Factor alpha inhibitor in COVID-19 outcomes: a prospective clinical trial study. | Iran | N | N | Drug Repurposing | Etanercept biosimilar | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201907 27044343N2 |
Atorvastatin effect in Clinical and Laboratory findings of patiens with COVID-19 admitted RAZI referral hospital in Mazandaran State: A Randomized Clinical Trial | Iran | Y | N | Drug Repurposing | Atorvastatin | Other | Mild/Moderate Cases, Severe Cases |
| IRCT201711 22037571N2 |
A single-arm multicenter clinical trial to evaluate the safety and efficacy of Remdesivir in COVID-19 patients with progressive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) | Iran | N | N | Shelved drug or NME | Remdesivir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202003 24046850N2 |
Comparison of the effect of Sofosbuvir + Daclatasvir (Sovodac) and Ribavirin in Covid-19 Patients with Severe Symptoms | Iran | Y | N | Drug Repurposing | Daclatasvir/sofosbuvir | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| IRCT201908 04044429N1 |
Efficacy and safety evaluation of therapeutic regimen of lopinavir/ritonavir and interferon beta 1b in patients with COVID-19 | Iran | Y | N | Drug Repurposing | Interferon beta-1b, Hydroxychloroquine, Lopinavir/ritonavir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202002 04046369N1 |
Evaluation of Methylprednisolone Administration as a Therapeutic Option in the 2019 Novel Coronavirus (COVID-19): A Non-Randomized Controlled Study | Iran | N | N | Drug Repurposing | Methylprednisolone | Efficacy - Oxygen parameters | Severe Cases |
| IRCT201308 12014333N145 |
Comparative assessment of the efficacy and safety of add ontreatment with “Sofosbuvir plus Velpatasvir” to “standard of care therapeutic regimen” in patients with COVID-19 | Iran | Y | N | Drug Repurposing | Sofosbuvir/velpatasvir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202003 25046859N1 |
Evaluation of the efficacy of intravenous immunoglobulin (IVIg) in patients with severe COVID-19 (Before intubation phase) who have not responded to treatment with the standard three-drug protocol (hydroxychloroquine / chloroquine + lupinavir / ritonavir + ribavirin) | Iran | N | N | Cell/Blood based | ND:Immunoglobulin | Efficacy - Other efficacy | Severe Cases |
| IRCT201612 04031229N3 |
Study of the Efficacy of Teicoplanin on mortality rate of patients with COVID-19 Infection: A randomized clinical trial | Iran | Y | N | Drug Repurposing | Teicoplanin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT201309 17014693N10 |
Evaluation the efficacy and safety of Hydroxychloroquine administration for COVID-19 post exposure prophylaxis | Iran | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Other efficacy | Exposed individuals (family members, HCP) |
| IRCT201308 12014333N147 |
Comparative assessment of the efficacy and safety of addon treatment with “Sofosbuvir-Daclatasvir”, “Lithium”,and “Trifluoprazine” to “standard of care in three groups of patients with COVID-19 | Iran | Y | Y | Drug Repurposing | Sofosbuvir-Daclatasvir, Lithium, Trifluoperazine, Trihexyphenidyl | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201312 15015805N2 |
Assessment of effect of levamisole on pneumonia caused by COVID-19 | Iran | Y | N | Drug Repurposing | Levamisole | Efficacy - Other efficacy | Mild/Moderate Cases |
| IRCT202004 03046926N1 |
The effect of adding SOVODAK (sofosbuvir+daclatasvir) to the treatment protocol of COVID-19 outpatients: A Clinical Trial Study | Iran | Y | N | Drug Repurposing | Sofosbuvir/daclatasvir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201512 28025732N53 |
Evaluation of the therapeutic effects of Convalescent Plasma (CP) of recovered people from Covid-19 in improving clinical and laboratory symptoms of hospitalized patients | Iran | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Oxygen parameters | Severe Cases |
| IRCT201303 06012728N8 |
Prospective post-exposure hydroxycloroquine prophylaxis for Covid 19 (PROSCLOROCOV 19) | Iran | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| IRCT201512 27025726N14 |
Evaluation the efficacy and safety of Favipiravir made by Shahid Beheshti University of Medical Sciences in comparison with Lopinavir-ritonavir in COVID-19 patients | Iran | Y | N | Drug Repurposing | Favipiravir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202004 05046958N1 |
Effect of Hydroxychloroquine on Novel Coronavirus Disease (COVID-19) prevention in cancer patients under treatment | Iran | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Other |
| IRCT202003 18046812N2 |
Safety and efficacy of “Hydroxychloroquine + Azithromycin + Naproxen + Prednisolone” and “Hydroxychloroquine + Azithromycin + Naproxen” regimens in comparison with “Hydroxychloroquine + kaletra” on the need for intensive care unit treatment in patients with COVID-19; a randomized, multicenter, parallel groups, open label study | Iran | Y | N | Drug Repurposing | Prednisolone | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT202003 17046797N4 |
Effect of Bromhexine Hydrochloride on clinical improvement and outcome of COVID-19-induced pneumonia | Iran | Y | N | Drug Repurposing | Bromhexine | Efficacy - Other efficacy | Mild/Moderate Cases |
| IRCT202004 04046948N1 |
Randomized, parallel-controlled and multi-center clinical study evaluating the efficacy and safety of convalescent plasma, in the treatment of patients with severe SARS-CoV-2 infection (COVID-19) | Iran | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - WHO scale | Severe Cases |
| IRCT201805 20039738N2 |
Comparison of the effectiveness of standard treatment with stand treatment plus Vitamin A in treatment in covid19 patients | Iran | Y | N | Drug Repurposing | Vitamin A | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT200810 19001369N4 |
Clinical trial of minocycline against COVID-19 | Iran | Y | N | Drug Repurposing | Minocycline | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202004 08046992N1 |
Evaluation of the Effect of Trifluoperazin in Treatment of Patients with Confirmed 2019 Novel Coronavirus(COVID- 19): An Open-Label Randomized Clinical Trial | Iran | Y | N | Drug Repurposing | Trifluoperazine | Efficacy - WHO scale | Severe Cases |
| IRCT202004 08046987N1 |
Dose-Finding study of Ivermectin treatment on patients infected with Covid-19:A clinical trial | Iran | Y | N | Drug Repurposing | Ivermectin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202003 17046797N3 |
To evaluate the effectiveness of intravenous immunoglobulin (IVIG) for the treatment of COVID-19-induced cytokine storm | Iran | Y | N | Cell/Blood based | ND:Immunoglobulin | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| IRCT202004 06046968N1 |
The effect of IL-6 inhibitor (Tocilizumab) on the prognosis of covid-19 patients with acute respiratory failure | Iran | N | N | Drug Repurposing | Tocilizumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201508 08023559N20 |
Evaluation of safety and efficacy of hydroxychloroquine plus favipiravir drug regimen in comparison with hydroxychloroquine plus kaletra in hospitalized patients with COVID-19 | Iran | Y | N | Drug Repurposing | Favipiravir | Efficacy - Mortality | Severe Cases |
| IRCT202004 10047009N1 |
Evaluation of the role of naproxen as adjunctive therapy with standard therapies and its efficacy in the early improvement and reduced mortality rate of COVID-19 patients | Iran | Y | N | Drug Repurposing | Naproxen | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202004 09047007N1 |
The effect of plasma administration of COVID-19 survivors in patients with acute respiratory distress syndrome due to COVID-19 | Iran | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases, Other |
| IRCT202002 17046526N2 |
Mesenchymal Stem Cell Therapy for Acute Respiratory Distress Syndrome in Coronavirus Infection: A Phase 2-3 Clinical Trial | Iran | Y | N | Cell/Blood based | ND:Stem cells | Safety | Mild/Moderate Cases, Severe Cases |
| IRCT202004 11047025N1 |
Evaluation of effectiveness? of Intravenous vitamin c in Patients with COVID-19 Referred to Imam ?Khomeini Hospital: a clinical trial ? | Iran | Y | N | Drug Repurposing | Ascorbic acid | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| IRCT200809 01001165N46 |
Investigating the efficacy and safety of Umifenovir in controlling the symptoms of patients with COVID-19 | Iran | Y | N | Drug Repurposing | Umifenovir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202004 06046963N1 |
Evaluation of the efficacy and safety of methylprednisolone pulse therapy in treatment of Covid-19 patients with ARDS | Iran | Y | N | Drug Repurposing | Methylprednisolone | Efficacy - Mortality | Severe Cases |
| IRCT201901 22042450N4 |
Prevention of COVID-19 disease after contact with an infected patient with coronavirus after taking hydroxychloroquine in the community | Iran | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Other efficacy | Exposed individuals (family members, HCP) |
| IRCT202004 11047030N1 |
Evaluation of adding melatonin to routine treatment on outcomes and quality of sleep in COVID-19 patients | Iran | N | N | Drug Repurposing | Melatonin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201907 17044241N2 |
Cell therapy in patients with coronavirus19 using mesenchymal stem cells | Iran | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202004 06046968N2 |
Efficacy of convalescent plasma transfusion of COVID-19 survivors on the treatment of respiratory failure of these patients | Iran | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Viral load | Severe Cases |
| IRCT201702 07032444N3 |
The efficacy and safety of Thalidomide in severe Covid19 pneumonia: Arandomized controlled clinical trial | Iran | Y | N | Drug Repurposing | Thalidomide | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| IRCT200810 19001369N5 |
Clinical trial of lithium in improving the clinical and laboratory symptoms of patients with COVID-19 | Iran | Y | N | Drug Repurposing | Lithium | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202004 08046988N1 |
Evaluation of the efficacy of melatonin tablets as auxiliary medication in accelerating the improvement of the COVID-19 symptoms and clinical findings: A double-blind randomized and placebo controlled trial | Iran | Y | N | Drug Repurposing | Melatonin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| IRCT202004 13047062N1 |
Evaluating the efficacy of Atorvastatin in clinical improvement, prognosis and hospitalization course in patients with COVID-19 infection | Iran | Y | N | Drug Repurposing | Atorvastatin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202004 06046968N3 |
effect of Interfron-beta1(zifron) on clinical improvement and prognosis of COVID-19 in Tabriz Imam-Reza hospital | Iran | Y | N | Drug Repurposing | Interferon beta-1b | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| IRCT202004 13047063N1 |
Placental Mesenchymal Stem cells for treatment of ARDS in Coronavirus infection, Phase 1 and 2 Clinical Trials | Iran | Y | N | Cell/Blood based | ND:Stem cells | Safety | Mild/Moderate Cases, Severe Cases |
| IRCT202004 14047070N1 |
Determining the effect of skin test purified protein derivative (PPD) on the recovery process of patients with COVID 19 in patients with this disease | Iran | Y | N | Cell/Blood based | ND:Purified protein derivative | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| IRCT201702 10032478N3 |
The efficacy of inhaled formoterol on symptom improvement in covid 19 patients | Iran | Y | N | Drug Repurposing | Formoterol | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202004 13047056N1 |
Comparison between the efficacy of intravenous immunoglobulin and convalescent plasma in improving the condition of patients with COVID-19: A randomized clinical trial | Iran | Y | N | Cell/Blood based | ND:Immunoglobulin, ND:Convalescent plasma | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT202004 18047116N1 |
Effect of Intravenous immunoglobulin (IVIG) versus Kaletra (lopinavir and ritonavir) tablets in patients with acute respiratory infection (COVID-19): A clinical trial studies | Iran | N | N | Cell/Blood based | ND:Immunoglobulin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202004 15047092N1 |
Safety and Effectiveness of azithromycin in Patients with COVID-19 Referred to zeiaeian Hospital : A clinical trial study | Iran | Y | N | Drug Repurposing | Azithromycin | Efficacy - Other efficacy | Severe Cases |
| IRCT202004 17047113N1 |
Evaluating the safety and efficacy of allogeneic NK cells on COVID-19 induced pneumonia, double blind, randomized clinical trial | Iran | Y | Y | Cell/Blood based | ND:NK-cells | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202004 19047128N1 |
Assessment of the effect of Tranilast on the efficacy of antiviral drug regimens in the treatment of patients with severe COVID19 | Iran | Y | N | Drug Repurposing | Tranilast | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| IRCT202004 16047099N1 |
Use of convalescent plasma in the treatment of patients with severe COVID-19 pneumonia | Iran | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Severe Cases |
| IRCT202004 18047122N1 |
Evaluation of the protective effect of a combination drug including Apocynin, Niacin, and Tannin on prevention of cardiovascular and respiratory morbidities and mortality in COVID-19 infection: A Clinical Trial | Iran | Y | N | Drug Repurposing | Niacin-Tannin, Apocynin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| IRCT202004 18047121N2 |
Investigation the adipose and placenta-derived mesenchymal stem cells effect on the respiratory distress syndrome in patients with COVID-19: a pilot study | Iran | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Infection rate (prevention) | Mild/Moderate Cases |
| IRCT202003 29046892N1 |
Evaluation of Trifluoperazine Effectiveness in Treatment Process, Survival rate and Cure rate of COVID-19 Patients | Iran | Y | N | Drug Repurposing | Trifluoperazine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201512 22025660N2 |
The prophylactic effect of Hydroxychloroquine on Novel Corona virus (COVID-19) in health care providers | Iran | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| IRCT202004 20047147N1 |
Evaluation the efficacy and safety of Sitagliptin administration in patients with COVID-19 | Iran | Y | N | Drug Repurposing | Sitagliptin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT202004 21047155N1 |
Therapeutic Effect of Tenofovir and hydroxychloroquine combination therapy compared to chloroquine alone in patients with COVID-19 | Iran | Y | N | Drug Repurposing | Tenofovir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202004 22047168N1 |
Comparison of the Effectiveness of Tenofovir antiviral drug beside (Kaletra and Chloroquine) with routine drug regime (Kaletra and Chloroquine) in COVID-19 patients | Iran | Y | N | Drug Repurposing | Tenofovir | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| IRCT202004 08046990N2 |
Evaluation of colchicine tablet efficacy as an adjuvant treatment for patients with mild to moderate COVID-19 in Qaem Hospital, Mashhad: A triple-blind randomized placebo controlled clinical trial | Iran | Y | N | Drug Repurposing | Colchicine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| IRCT201801 14038350N3 |
Investigating the effect of Deferiprone on the improvement of symptoms of Coronavirus 2019 (COVID 19) | Iran | Y | N | Drug Repurposing | Deferiprone | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201202 15009014N353 |
Effect of plasma of patients recovered from covid-19 versus control group on treatment of covid-19: a randomized clinical trial | Iran | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201403 05016852N4 |
Comparison of three methods of treatment in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with and without coronavirus positive test (Kovid-19) | Iran | Y | N | Drug Repurposing | Ascorbic acid, Vitamin D3 | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201804 25039414N2 |
The effect of zinc on the treatment and clinical course of patients with SARS-cov2 (COVID-19) | Iran | Y | N | Drug Repurposing | Zinc | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202004 27047215N1 |
Efficacy and safety of oral indomethacin for treatment of covid 19 induced pneumonia | Iran | Y | N | Drug Repurposing | Indomethacin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT200809 01001165N50 |
Investigating the efficacy and safety of Azithromycin inhaled spray in controlling the symptoms of patients with COVID-19 | Iran | Y | N | Drug Repurposing | Azithromycin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| IRCT200809 01001165N51 |
Investigating the efficacy and safety of Hydroxychloroquine nasal spray in controlling the symptoms of patients with COVID-19 | Iran | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| IRCT202004 18047121N1 |
Study of the effectiveness of concomitant use of vitamin C, metformin, azithromycin and doxycycline on improving patients with COVID-19 | Iran | Y | N | Drug Repurposing | Azithromycin, Doxycycline, Ascorbic acid, Metformin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201508 08023559N21 |
The Effect of Convalescent Plasma Therapy on the Outcomes of Patients with 19-COVID | Iran | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| IRCT202005 01047258N1 |
Investigation of the effects of COVID-19 convalescent plasma in acute respiratory distress syndrome due to COVID-19 | Iran | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Infection rate (prevention) | Mild/Moderate Cases, Severe Cases |
| IRCT200809 01001165N52 |
Investigating the efficacy of high dose of glucocorticoid in patients with moderate to severe pneumonia related to COVID-19 | Iran | Y | N | Drug Repurposing | Methylprednisolone, Prednisolone | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT202005 08047346N1 |
Evaluation of the efficacy and safety of Rabbit polyclonal antibody (CoviGlobulin) in patients with coronavirus COVID-19 virus moderate to severe | Iran | Y | N | Cell/Blood based | ND:CoviGlobulin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201202 15009014N354 |
Evaluating the effect of intravenous hydrocortisone, methylprednisolone, and dexamethasone in treatment of patients with moderate to severe acute respiratory distress syndrome caused by COVID-19: A double blind randomized clinical trial | Iran | Y | Y | Drug Repurposing | Hydrocortisone, Methylprednisolone, Dexamethasone | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| KCT0005003 | Treatment effect of Nafamostat mesilate in patients with COVID-19 pneumonia: open labelled randomized controlled clinical trial | Korea, Republic of | Y | N | Drug Repurposing | Nafamostat | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| ACTRN12620 000445976 |
Australasian COVID-19 Trial (ASCOT). A multi-centre randomised clinical trial to assess clinical, virological and immunological outcomes in patients with SARS-CoV-2 infection (COVID-19) treated with lopinavir/ritonavir and/or hydroxychloroquine compared to standard of care | Australia | Y | N | Drug Repurposing | Hydroxychloroquine, Lopinavir/ritonavir | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| ACTRN12620 000580976 |
Tocilizumab for the treatment of COVID-19 in intensive care patients: effect on days free of ventilatory support. | Australia | N | N | Drug Repurposing | Tocilizumab | Efficacy - Mortality | Severe Cases |
| ISRCTN8521 6856 |
Investigating the use of convalescent plasma from patients who have recovered from COVID-19 in the management of Ecuadorian patients infected with SARS-CoV-2 with clinical deterioration: IMPACT | Ecuador | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 033056 |
Convalescent Plasma in the treatment of 6 COVID-19 Patients | China | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 033049 |
A clinical study for the efficacy and safety of artemisinin-pipequine tablets in the treatment of the untreated mild or common type novel coronavirus pneumonia (COVID-19) patients | China | N | N | Drug Repurposing | Artemisinin-piperaquine | Efficacy - Viral load | Mild/Moderate Cases |
| ChiCTR2000 033372 |
Efficacy and safety of leflunomide for Refractory novel coronavirus pneumonia (COVID-19): A non-Randomized Controlled Study | China | N | N | Drug Repurposing | Leflunomide | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 033323 |
Severe Reluctant COVID-19 Patients Responding to Convalescent Plasma; A case series | Iraq | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 033627 |
In vivo use of ivermectin (IVR) for treatment for corona virus infected patients: a randomized controlled trial | Iraq | Y | N | Drug Repurposing | Ivermectin | Efficacy - Viral load | Mild/Moderate Cases |
| ChiCTR2000 033491 |
The Single Oral Favipiravir for Patients with Delayed SARS-Cov-2 viral RNA Clearance | China | N | N | Drug Repurposing | Favipiravir | Efficacy - Viral load | Mild/Moderate Cases |
| ChiCTR2000 033774 |
Evaluating the Efficacy and Safety of Bromhexine Hydrochloride Tablets in Treating Pediatric novel coronavirus pneumonia (COVID-19) | China | Y | N | Drug Repurposing | Bromhexine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Other |
| IRCT202001 28046294N2 |
A prospective randomized controlled trial comparing Sovodak (Sofosbuvir plus Daclatasvir) in participants with moderate to severe Coronavirus disease (COVID-19) compared to standard of care treatment | Iran | Y | N | Drug Repurposing | Daclatasvir/sofosbuvir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202003 14046774N1 |
Assessment of vitamin A effectiveness on COVID-19 patients and treatment outcomes referred to health centers of Qom province | Iran | Y | N | Drug Repurposing | Vitamin A | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| IRCT202003 24046850N3 |
The effect of naproxen on the healing process of patients with COVID-19 | Iran | Y | N | Drug Repurposing | Naproxen | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT202003 24046850N1 |
Comparison of vitamin D3 and N-acetylcysteine prescription in COVID19 patients and their effect on recovery process | Iran | Y | Y | Drug Repurposing | Vitamin D3, N-acetylcysteine | Efficacy - Other efficacy | Severe Cases |
| IRCT202003 18046812N1 |
Evaluation of safety and efficacy of hydroxychloroquine plus favipiravir drug regimen in comparison with hydroxychloroquine plus kaletra on the need for intensive care unit treatment in patients with COVID-19; a randomized, multicenter, parallel groups, open label study | Iran | Y | N | Drug Repurposing | Favipiravir, Lopinavir/Ritonavir | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT201908 10044500N5 |
Investigation of the efficacy and safety of colchicine In combination with standard treatment on covid-19 patients: A clinical trial | Iran | Y | N | Drug Repurposing | Colchicine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202004 01046909N1 |
The efficacy of oral 25-hydroxyvitamin D3 on COVID-19 treatment in adults: A Randomized, Controlled Double-Blind Clinical Trial. | Iran | Y | N | Drug Repurposing | Vitamin D3 | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| IRCT201807 25040596N2 |
Evaluation of the effect of Arbidol drug in the treatment of hospitalized patients with COVID-19 | Iran | Y | N | Drug Repurposing | Umifenovir, Lopinavir/ritonavir | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| IRCT202004 04046947N1 |
Study of Methylprednisolone effects on treatment and clinical symptoms and laboratory signs of Iranian ?COVID-19 patients: a clinical trial study | Iran | Y | N | Drug Repurposing | Methylprednisolone | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| IRCT201512 28025732N52 |
A study on melatonin and Vitamin C and zinc efficacy in patients with COVID19 hospitalized in intensive care unit of Semnan Kowsar Hopsital | Iran | Y | N | Drug Repurposing, Other | ND:Zinc, Ascorbic acid | Efficacy - Oxygen parameters | Severe Cases |
| IRCT200810 27001411N3 |
Study of Prednisolone effects on treatment and clinical symptoms and laboratory signs of Iranian COVID-19 patients: a clinical trial study | Iran | Y | N | Drug Repurposing | Methylprednisolone | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT200912 01002804N12 |
The effect of surfactant on clinical outcome of patients with COVID-19 under mechanical ventilation | Iran | Y | N | Drug Repurposing | Beractant | Efficacy - Oxygen parameters | Severe Cases |
| IRCT202004 01046909N2 |
Investigating preventive effects of oral 25-hydroxyvitamin D3 on COVID-19 in adults: A Randomized, Controlled Double-Blind Clinical Trial. | Iran | Y | N | Drug Repurposing | Vitamin D3 | Efficacy - Infection rate (prevention) | General population |
| IRCT202002 17046526N1 |
Mesenchymal Stem Cell Therapy for Acute Respiratory Distress Syndrome in Coronavirus Infection : A Phase 1 and 2 clinical trial | Iran | N | N | Cell/Blood based | ND:Stem cells | Safety | Mild/Moderate Cases, Severe Cases |
| IRCT201907 27044343N3 |
Evaluation of ANIF1 antiviral drug in COVID-19 patients: A Randomized Clinical Trial | Iran | Y | N | Shelved drug or NME | ANIF1 | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| IRCT200810 19001369N6 |
Evaluation of the effect of trans sodium crocetinate in respiratory distress caused by COVID-19 | Iran | Y | N | Shelved drug or NME | Trans sodium crocetinate | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| IRCT200910 12002582N21 |
Effect of intratracheal Injection of Processed Autologous Serum Derived from Patients with covid-19 in oxygenation parameters and pulmonary Complications | Iran | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Oxygen parameters | Severe Cases |
| IRCT202003 25046859N2 |
evaluation of the effects of the 2-drug diet (hydroxychloroquine + umifenovir(Arbidol)) compared with Hydroxy chlorquine on the of mortality rate in patients with moderate symptoms of COVID-19 infections: A randomized interventional study in Imam Reza Hospital, Mashhad. | Iran | N | N | Drug Repurposing | Umifenovir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| IRCT202004 11047019N1 |
Investigating the Effect of BCG Vaccine on Preventing COVID-19 Infection in Healthcare Staff Exposed to SARS-CoV-2 | Iran | Y | N | Vaccine | BCG Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| IRCT201503 03021315N19 |
The evaluation of safety and efficacy of Pembrolizumab (CinnaGen Co, Iran) in patients with COVID-19 | Iran | N | N | Drug Repurposing | Pembrolizumab | Efficacy - Mortality | Mild/Moderate Cases |
| IRCT202005 01047259N1 |
Intravenous immunoglubolin (IVIG) effect on improvement of severe pulmonary damage in COVID 19 disease | Iran | Y | N | Cell/Blood based | ND:Immunoglobulin | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| IRCT202005 02047268N1 |
Evaluation of the effect of GCSF injection in treatment of COVID-19 patients with lymphopenia (less than 1000) | Iran | Y | N | Drug Repurposing | Granulocyte colony-stimulating factor | Efficacy - Other efficacy | Other |
| IRCT202004 18047126N1 |
Evaluation of the therapeutic effect of colchicine plus chloroquine in comparison with chloroquine in patients with COVID-19 | Iran | Y | N | Drug Repurposing | Colchicine | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT202004 05046951N1 |
The effect of supplementation with vitamin A on improvement of symptoms of acute respiratory syndrome in patients with COVID-19: A randomized clinical trial | Iran | Y | N | Drug Repurposing | Vitamin A | Efficacy - Oxygen parameters | Severe Cases |
| IRCT202004 21047150N1 |
Assessment of safety, efficacy and effective dose determination of human umbilical cord Wharton’s jelly mesenchymal stem cell transplantation on treatment of COVID-19 (coronavirus) pneumonia and complications in humans | Iran | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| IRCT201404 28017469N1 |
Short-term Protection for COVID-19 by Oral Polio Vaccine | Iran | N | N | Vaccine | ND:Polio vaccine | Efficacy - Infection rate (prevention) | General population, Other |
| IRCT201512 27025726N15 |
Evaluation the efficacy and safety of Umifenovir (Arbidol) Administration in comparison with Lopinavir-ritonavir (Kaletra) in COVID-19 patients | Iran | Y | N | Drug Repurposing | Umifenovir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| IRCT201507 16023235N15 |
The use of sex hormones in the control of coronavirus inflammation | Iran | Y | N | Drug Repurposing | Testosterone, Progesterone | Efficacy - Clinical improvement (except WHO scale) | Other |
| IRCT202005 10047383N1 |
Evaluation of the effect of Tocilizumab on outcomes of the severe COVID-19, determining indications within the paradigm of host-directed therapy | Iran | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Mortality | Severe Cases |
| IRCT201202 15009014N355 |
Evaluating the effect of intravenous N-acetyl cysteine versus placebo in the treatment of patients with mild and moderate acute respiratory distress syndrome caused by COVID-19: A double-blind randomized clinical trial | Iran | Y | N | Drug Repurposing | N-acetylcysteine | Efficacy - Mortality | Mild/Moderate Cases |
| IRCT201707 31035423N2 |
Effect of the combination of BCc1 & Hep-S on the improvement of clinical & laboratory symptoms of hospitalized COVID-19 patients in a randomized, double-blind, clinical trial | Iran | Y | N | Shelved drug or NME | BCc1 nanomedicine, Hep-s | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT200809 01001165N55 |
Investigating the efficacy and safety of N-Acetyl Cysteine (NAC) inhalation spray in controlling the symptoms of patients with COVID-19 | Iran | Y | N | Drug Repurposing | N-acetylcysteine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT200809 01001165N53 |
Investigating the efficacy and safety of Interferon Beta1a nasal spray in controlling the symptoms of patients with COVID-19 | Iran | Y | N | Drug Repurposing | Interferon Beta-1A | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201512 27025726N17 |
Evolution of the efficacy and safety of Dexamethasone administration in patients with mild to moderate COVID-19 acute respiratory disease syndrome | Iran | Y | N | Drug Repurposing | Dexamethasone | Efficacy - Oxygen parameters | Mild/Moderate Cases |
| IRCT202005 16047468N1 |
Interventional study of intravenous vitamin C in definitive patients with covid 19 and its effect on changes in lung CT scan and clinical and laboratory symptoms of patients | Iran | Y | N | Drug Repurposing | Ascorbic acid | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT201501 07020592N23 |
Plasmapheresis and plasma exchanges;usefulness in covid-19 critically ill patients | Iran | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Oxygen parameters | Severe Cases |
| IRCT201608 09029275N1 |
Evolution of Allogenic Mesenchymal stem cell- derived Umbilical cord transplantation for ARDS patients infected with COVID19. | Iran | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Oxygen parameters | Severe Cases |
| IRCT202005 17047485N1 |
Evaluation of the efficacy and side effects of combination therapy of Kaletra and Hydroxychloroquine in comparison with Atazanavir / Ritonavir and Hydroxychloroquine in the treatment of patients with COVID-19 | Iran | Y | N | Drug Repurposing | Atazanavir/ritonavir, Lopinavir/ritonavir | Efficacy - Oxygen parameters | Mild/Moderate Cases |
| IRCT201501 07020592N22 |
Cyclosporin for the Prevention and Treatment of COVID-19 Disease | Iran | N | N | Drug Repurposing | Cyclosporine | Efficacy - Other efficacy | Severe Cases |
| IRCT201512 27025726N18 |
The effect of pentaglobin in the treatment of critically ill patients with COVID-19 | Iran | Y | N | Cell/Blood based | ND:Immunoglobulin | Efficacy - Oxygen parameters | Severe Cases |
| IRCT201612 06031256N3 |
Evaluation and comparison of the effect of two interferon alpha and beta antiviral drugs on the prognosis of patients with COVID 19 | Iran | Y | Y | Drug Repurposing | Interferon alpha, Interferon beta | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT202004 22047168N2 |
A randomized clinical trial study, comparison of the therapeutic effects of Ivermectin, Kaletra and Chloroquine with Kaletra and Chloroquine in the treatment of patients with coronavirus 2019 (COVID-19) | Iran | Y | N | Drug Repurposing | Ivermectin | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| IRCT202005 23047550N1 |
Evaluating effectiveness and safety of Umifenovir in the treatment (COVID-19)infection In patients referred to Tehran Imam Khomeini Hospital Complex | Iran | N | N | Drug Repurposing | Umifenovir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201601 18026097N3 |
Evaluation of interferon treatment in high-risk covid19 patients in Qom | Iran | Y | N | Drug Repurposing | Interferon beta-1b | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| IRCT201207 03010178N20 |
?Evaluation of Safety and efficacy of anakinra utilization in COVID-19, a randomized controlled clinical trial | Iran | Y | N | Drug Repurposing | Anakinra | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| IRCT202004 12047042N1 |
Evaluation the effect of raltegravir, and raltegravir/interferon beta combination on covid 19 patients admitted in Peymanieh hospital of Jahrom in 2020 | Iran | Y | Y | Drug Repurposing | Raltagravir, Interferon beta | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| JPRN-Japic CTI-205270 |
A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia in Japan | Japan | N | N | Drug Repurposing | Tocilizumab | Safety | Severe Cases |
| JPRN-jRCTs 031190227 |
A prospective multi-center open trial to evaluate the safety and efficacy of triple combination therapy of lopinavir , ritonavir and hydroxychloroquine sulfate in patients infected with COVID-19. | Japan | N | N | Drug Repurposing | Hydroxychloroquine, Lopinavir/ritonavir | Efficacy - Other efficacy | Mild/Moderate Cases |
| JPRN-jRCTs 041190120 |
Multicenter, open-label, randomized trial of favipiravir in asymptomatic and minimally symptomatic patients infected with SARS-CoV2 to evaluate viral load reduction | Japan | Y | N | Drug Repurposing | Favipiravir | Efficacy - Viral load | Mild/Moderate Cases |
| JPRN-jRCTs 061200002 |
Randomized controlled trial of teprenone for preventing aggravation of COVID-19 | Japan | Y | N | Drug Repurposing | Teprenone | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| ACTRN12620 000501943 |
Effectiveness of Prophylactic Hydroxychloroquine on incidence of COVID-19 infection in Front-line Health and Allied Health Care Workers: The COVID-SHIELD Trial | Australia | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| ACTRN12620 000676910 |
Allogeneic Amniotic Epithelial Cells for the Treatment of COVID-19 related respiratory failure, a pilot feasibility randomised controlled trial | Australia | Y | N | Cell/Blood based | ND:Amniotic epithelial cells | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| ISRCTN3357 8935 |
Rationale and investigational study for the treatment of COVID-19 with severe viral pneumonia with isolated, placental, mesenchymal stem cell exosomes | Germany | Y | N | Cell/Blood based | ND:Stem cell exosomes | Safety | Severe Cases |
| ISRCTN4030 2986 |
A double-blind clinical trial to repurpose and assess the efficacy and safety of ivermectin in COVID-19 | Nigeria | Y | N | Drug Repurposing | Ivermectin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 034112 |
A Phase I clinical trial to evaluate the safety, tolerance and preliminary immunogenicity of different doses of a SARS-CoV-2 mRNA vaccine in population aged 18-59 years and 60 years and above | China | Y | Y | Vaccine | ND:Vaccine | Safety | Other |
| ChiCTR2000 034076 |
A safety and immune response trial of novel coronavirus pneumonia (COVID-19) S-Vax loaded autologous dendritic cells | China | N | N | Vaccine | ND:Vaccine | Safety | Other |
| ACTRN12620 000707965 |
A Multicenter, Phase III, Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Efficacy of the recombinant BCG VPM1002 on the Incidence or Disease Severity of SARS-COV-2/COVID-19 Among High-Risk Participants in Australia | Australia | Y | N | Vaccine | ND: VPM1002 vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| ACTRN12620 000612910 |
A pilot, open-label, randomised controlled clinical trial to investigate early efficacy of CYP-001 in adults admitted to intensive care with COVID-19 | Australia | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Oxygen parameters | Severe Cases |
| SLCTR/2020 /011 |
Hydroxychloroquine for Post-Exposure Prophylaxis of COVID-19 among naval personnel: a placebo-controlled, randomized, clinical trial | Sri Lanka | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| IRCT201511 13025025N3 |
Clinical Trial of renin-angiotensin-aldosterone system inhibitors with halting their administration and the effect on clinical outcomes of patients with coronavirus disease-2019 (COVID-19) referring to Sina and Imam-Khomeini Hospitals in 2020 | Iran | N | N | Drug Repurposing, Other | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases | |
| IRCT202004 16047099N2 |
Plasma exchange in patients with COVID-19 to reduce viral load and inflammatory molecules | Iran | N | N | Cell/Blood based | ND:Plasma exchange | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT202004 28047232N1 |
Effectiveness and safety of thalidomide in moderate COVID-19 pneumonia: A randomized clinical trial | Iran | Y | N | Drug Repurposing | Thalidomide | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| IRCT201711 05037262N4 |
Evaluation of adalimumab effect on clinical symptoms including respiratory distress, oxygen saturation and lung involvement of patients with COVID-19 | Iran | Y | N | Drug Repurposing | Adalimumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202005 11047396N1 |
Efficacy evaluation of inhalation therapy (nasal spray) of Interferon Beta-1a in hospitalized Covid-19 patients | Iran | Y | N | Drug Repurposing | Interferon Beta-1a | Efficacy - Other efficacy | Mild/Moderate Cases |
| IRCT201804 25039414N3 |
The effect of vitamin C and E in the treatment and clinical course of patients with SARS-cov2 (COVID-19) | Iran | Y | N | Drug Repurposing | Ascorbic acid, Vitamin E | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202005 10047385N1 |
Effect of stromal vascular fraction (SVF), blood and condition medium derived exosomes on treatment of covid-19 patients with Acute Respiratory Distress Syndrome(ARDS)/ clinical Trial | Iran | Y | Y | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT202005 13047426N1 |
The prophylactic effect of oral hydroxy-chloroquine in close contacts of COVID-19 patients | Iran | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| IRCT201107 26007117N11 |
Effect of vitamin D supplementation in diagnosed cases of 2019 Novel Coronavirus; a randomized clinical trial | Iran | Y | N | Drug Repurposing | Vitamin D3 | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT202001 12046089N1 |
Evaluation of the effect of Resveratrol on the effectiveness of antiviral drug regimen in patients with COVID-19 | Iran | Y | N | Shelved drug or NME | Resveratrol | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT200810 27001411N4 |
Study of Tocilizumab effect on treatment and clinical symptoms and laboratory signs of Iranian COVID-19 patients: a ?crinical trial study | Iran | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT202005 25047562N1 |
Immediate uses of convalescent covid-19 plasma in the treatment of new infected patients at the first day of hospitalization in a clinical trail | Iran | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| IRCT202003 17046797N6 |
Effect of edaravone on clinical improvement and outcome of patients with respiratory distress syndrome caused by COVID-19 | Iran | Y | N | Drug Repurposing | Edaravone | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT202005 04047298N1 |
Evaluation of Atazanavir/Ritonavir effect on COViD-19 patients treatment in comparison with Lopinavir / Ritonavir | Iran | Y | N | Drug Repurposing | Atazanavir, Ritonavir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202006 08047686N1 |
Evaluation of therapeutic effects of Metronidazole In Inpatients with Pneuomonia Due to COVID-19 | Iran | Y | N | Drug Repurposing | Metronidazole | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| IRCT201501 07020592N26 |
Favipiravir effectiveness in patients undergoing COVID19 Acute respiratory distress syndrome (ARDS) | Iran | N | N | Drug Repurposing | Favipiravir | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| IRCT202006 09047707N1 |
Effective of Elman tablet on illness severity and length of hospital stay in the patients with COVID-19 infection | Iran | Y | N | Shelved drug or NME | Elman | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT201501 07020592N27 |
The effect of pentaglobin in the treatment of critically ill patients with COVID-19 | Iran | N | N | Shelved drug or NME | Pentaglobin | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| IRCT202005 15047456N1 |
Evaluation of Tissue Plasminogen Activator (tPA) in comparition of anticoagulation for treatment of critical COVID 19 patient | Iran | Y | N | Drug Repurposing | Alteplase | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| IRCT201501 07020592N30 |
Favipiravir prophylactic to reduce the involvement of health care workers in the COVID-19 epidemic | Iran | N | N | Drug Repurposing | Favipiravir | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| IRCT201507 25023332N3 |
The effect of Bromelain and Montelukast on Clinical and non-Clinical parameters in Covid-19 patients | Iran | Y | Y | Drug Repurposing, Other | Montelukast, ND:Bromelain | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| IRCT201901 21042444N3 |
Considering the administration of Desferral supplementary and Vitamin C on the ferritin levels of Quid 19 patients | Iran | Y | N | Drug Repurposing | Deferoxamine, Ascorbic acid | Efficacy - Other efficacy | Mild/Moderate Cases |
| IRCT201112 24008507N3 |
Effectiveness of Ivermectin in the Treatment of Coronavirus Infection in Patients admitted to Educational Hospitals of Mazandaran in 2020 | Iran | Y | N | Drug Repurposing | Ivermectin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202006 24047908N1 |
Evaluation of the effect of sofosbuvir/daclatasvir in COVID-19 patients: A randomized double-blind clinical trial | Iran | Y | N | Drug Repurposing | Sofosbuvir/Daclatasvir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| IRCT202004 26047206N2 |
Clinical trial of efficacy and safety of mesenchymal stem cell transplantation in patients with COVID-19 pneumonia | Iran | Y | N | Drug Repurposing | ND:Stem cells | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| JPRN-jRCT2 031200035 |
A Multicenter, Adaptive, Randomized Blinded Controlled Trial of the Safety and Efficacy of Investigational Therapeutics for the Treatment of COVID-19 in Hospitalized Adults - Adaptive COVID-19 Treatment Trial (ACTT-2) | Japan | Y | N | Drug Repurposing, Shelved drug or NME | Baricitinib, Remdesivir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| JPRN-jRCTs 031190226 |
A prospective multi-center open trial to evaluate the safety and efficacy of favipiravir in patients infected with COVID-19. | Japan | N | N | Drug Repurposing | Favipiravir | Efficacy - Other efficacy | Mild/Moderate Cases |
| JPRN-jRCTs 031200026 |
Multicenter, Single blinded Randomized Controlled, Comparative Study to Evaluate the Efficacy and Safety of Favipiravir and Nafamostat Mesilate in Patients with COVID-19 Pneumonia | Japan | Y | N | Drug Repurposing | Favipiravir, Nafamostat | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| JPRN-jRCTs 041200025 |
Japanese, multicenter, phase II trial of combination therapy with favipiravir and corticosteroids for coronavirus disease 2019 (COVID-19) patients with mild respiratory failure - J-CRITICAL trial | Japan | N | N | Drug Repurposing | Favipiravir, Methylprednisolone | Efficacy - Other efficacy | Mild/Moderate Cases |
| PACTR20200 4801273802 |
A Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial of the Efficacy and Safety of Chloroquine Phosphate, Hydroxychloroquine sulphate and Lopinavir/Ritonavir for the Treatment of COVID-19 in Lagos State. | Nigeria | Y | Y | Drug Repurposing | Chloroquine, Hydroxychloroquine, Lopinavir/Ritonavir | Efficacy - WHO scale | Severe Cases |
| PACTR20200 5599385499 |
The host Epigenetic methylation repair in COVID-19 using Folic acid: A new suggested prevention and treatment. | Egypt | N | N | Drug Repurposing | Folic acid | Efficacy - Viral load | Mild/Moderate Cases |
| PACTR20200 4893013257 |
PROTECT-Surg | Ghana | Y | Y | Drug Repurposing | Hydroxychloroquine, Lopinavir/Ritonavir | Efficacy - Other efficacy | Other |
| PACTR20200 5681895696 |
A phase Ib/II single-blinded, randomised, controlled study to determine safety, immunogenicity and efficacy of the candidate Coronavirus Disease (COVID-19) vaccine ChAdOx1 nCoV-19 in adults in Kenya | Kenya | Y | N | Vaccine | ND: Vaccine ChAdOx1 | Safety | General population |
| PACTR20200 5622389003 |
Efficacy and safety of Hydroxychloroquine, Azythromycine and Zinc for the treatment of patients with SARS-Cov2 infection in Senegal: a dose ranging randomised trial. COVID-19 | Senegal | Y | N | Drug Repurposing | Hydrochloroquine, Azythromycine, Zinc | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| PACTR20200 6899597082 |
Randomized clinical trial, phase 2, to evaluate the efficacy of Artesunate IV alone or combined with vitamin C IV for the treatment of COVID-19 | Madagascar | Y | Y | Drug Repurposing | Artesunate, Ascorbic acid | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| PACTR20200 6537901307 |
An open-label, multicentre, randomised, adaptive platform trial of the safety and efficacy of several therapies, including antiviral therapies, versus control in mild/moderate cases of COVID-19 | Switzerland | Y | N | Drug Repurposing | Lopinavir/Ritonavir, Hydroxychloroquine | Efficacy - Oxygen parameters | Mild/Moderate Cases |
| PACTR20200 7606032743 |
Nebulized heparin in patients with mainly moderate coronavirus disease 2019: Randomized controlled trial. COVID-19 | Egypt | Y | N | Drug Repurposing | Heparin | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| PACTR20200 6922165132 |
COVID-19: An Adaptive Phase I/II Randomized Placebo-controlled Trial to Determine Safety, Immunogenicity and Efficacy of Non-replicating ChAdOx1 SARSCoV-2 Vaccine in South African Adults Living Without HIV; and Safety and Immunogenicity in Adults Living With HIV. | South Africa | Y | Y | Vaccine | ND:ChAdOx1 vaccine | Safety | General population, Other |
| PACTR20200 6760881890 |
A Multi-Center, Randomized, Double-Blind, Placebo- Controlled Clinical Trial of the Safety and Efficacy of Convalescent Plasma for the Treatment of COVID-19 in Hospitalized Patients in Lagos State | Nigeria | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Viral load | Severe Cases, Other |
| PACTR20200 6883882711 |
Effectiveness of Measles, Mumps and Rubella Vaccine in Health Care Professionals for COVID-19 | Egypt | Y | N | Drug Repurposing, Vaccine | ND: MMR vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| RBR-8969zg | Clinical Trial using N-acetylcysteine for treatment of 2019-nCoV Pneumonia | Brazil | Y | N | Drug Repurposing | N-acetylcysteine | Efficacy - Mortality | Severe Cases |
| RBR-3k4wxb | Evaluation of the use of Hydroxychlorochine in patients with discrete form of Covid-19: randomized clinical trial | Brazil | Y | Y | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| RBR-4vm3yy | Use of convalescent plasma submitted to pathogen inactivation for the treatment of patients with severe COVID-19 | Brazil | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| RBR-949z6v | Full versus prophylactic heparinization for the treatment of severe forms of SARS-Covid-19: clinical, randomized, open and controlled study - HeSAcovid trial | Brazil | Y | N | Drug Repurposing | Enoxaparin | Efficacy - Oxygen parameters | Severe Cases |
| RBR-876qb5 | Eculizumab for the treatment of Covid-19 severe cases - : Eculizumabe for COVID | Brazil | N | N | Drug Repurposing | Eculizumab | Efficacy - Mortality | Severe Cases |
| RBR-3zdynp | Clinical Characterisation Protocol for Severe Emerging Infections ISARIC/WHO: COVID-19 | Brazil | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| RBR-7jqpnw | Therapeutic effectiveness of COVID-19 convalescent plasma produced by HEMOPE: a multicenter, randomized and controlled clinical trial - PLAVID-TRIAL: Covid convalescent plasma - Clinical Trial | Brazil | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| RBR-5vpyh4 | Use of Anti-IL-17 in patients with the severe acute respiratory syndrome (SARS) associated with Covid-19 | Brazil | Y | N | Drug Repurposing | Secuquinumab | Efficacy - Other efficacy | Severe Cases |
| RBR-3rywwg | A phase IIb, double-blind, randomized controlled trial to evaluate the efficacy and safety of Naproxen compared to placebo in combination with Azithromycin or Levofloxacin in patients with Severe Acute Respiratory Syndrome during the Covid-19 pandemic | Brazil | Y | N | Drug Repurposing | Naproxen | Efficacy - Mortality | Severe Cases |
| TCTR202005 14001 |
An Investigation of the Efficacy and Safety of Favipiravir in COVID-19 Patients without Pneumonia â?? An open-label randomized controlled study â?? | Thailand | Y | N | Drug Repurposing | Favipiravir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| TCTR202004 04004 |
Comparative effectiveness of chloroquine and vitamin C prophylaxis in household contacts of confirmed COVID-19 patients | Thailand | Y | N | Drug Repurposing | Chloroquine, Ascorbic acid | Efficacy - Viral load | Mild/Moderate Cases |
| PER-013-20 | CONVALESCENT PLASMA USE AS TREATMENT FOR HOSPITALIZED PATIENTS WITH COVID-19 IN ESSALUD | Peru | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| PER-016-20 | PERUCONPLASMA: RANDOMIZED CLINICAL TRIAL TO EVALUATE SAFETY AND EFFICACY OF THE USE OF CONVALESCENT PLASMA IN HOSPITALIZED PATIENTS WITH COVID-19 | Peru | Y | N | Cell/Blood based | ND:Convalescent plasma | Safety | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 03/024402 |
Hydroxy Chloroquine, in open labelled, Randomised intervention for prevention of new infection and adverse outcomes following COVID-19 infection- A Tertiary Hospital based study - CORONA study | India | N | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| CTRI/2020/ 04/024479 |
Open labelled Randomised controlled trial to study the effect of Chloroquine in addition to standard therapy in COVID-19 patients | India | Y | N | Drug Repurposing | Chloroquine | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 04/024729 |
Topical Chloroquine Nasal Drops in Early Stage Covid 19- Impact on Viral load and cure rates | India | Y | N | Drug Repurposing | Chloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| CTRI/2020/ 04/024775 |
A Phase II, Open Label, Randomized Controlled Trial to Assess the Safety and Efficacy of Convalescent Plasma to Limit COVID-19 Associated Complications in Moderate Disease. - PLACID | India | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Mild/Moderate Cases |
| CTRI/2020/ 04/024749 |
A Multicenter, Phase III, Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Efficacy of Recombinant BCG VPM1002 in Reducing Infection Incidence and Disease Severity of SARS-COV-2/COVID-19 Among High-Risk Subjects | India | Y | N | Drug Repurposing | BCG Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| CTRI/2020/ 04/024806 |
Efficacy of Imatinib in mild SARS CoV2 infection: A randomized study | India | Y | N | Drug Repurposing | Imatinib | Efficacy - Viral load | Mild/Moderate Cases |
| CTRI/2020/ 04/024804 |
Open Label, parallel arm, phase I/II clinical trial to evaluate Safety and efficacy of Convalescent Plasma as Therapy for Covid-19 Severe SARS-CoV-2 Disease | India | N | N | Cell/Blood based | ND:Convalescent plasma | Safety | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 04/024833 |
Effect of BCG-Denmark (Green Signal) on prevention of COVID 19 infection in health care workers â?? a double blind randomized controlled trial | India | Y | N | Drug Repurposing | BCG Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| CTRI/2020/ 04/024846 |
A clinical trial to evaluate the safety and efficacy of Mycobacterium W in critically ill patients suffering from COVID 19 infection | India | Y | N | Shelved drug or NME | ND:Mycobacterium | Efficacy - Other efficacy | Severe Cases |
| CTRI/2020/ 04/024904 |
Randomised Controlled Trial to compare efficacy of hydroxychloroquine alone and in combination with azithromycin in treatment of COVID-19 - HAZES | India | Y | Y | Drug Repurposing | Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 04/024948 |
EFFICACY OF HYDROXYCHLOROQUINE, CICLESONIDE AND IVERMECTIN IN TREATMENT OF MODERATE COVID-19 ILLNESS: AN OPEN-LABEL RANDOMISED CONTROLLED STUDY - EHYCIVER-COVID | India | Y | N | Drug Repurposing | Hydroxychloroquine, Ciclesonide, Ivermectin | Efficacy - Viral load | Mild/Moderate Cases |
| CTRI/2020/ 04/024949 |
EFFICACY OF ORAL NICLOSAMIDE IN TREATMENT OF MILD AND VERY MILD COVID-19 CASES: AN OPEN-LABEL RANDOMIZED CONTROLLED TRIAL - EONIC-COVID | India | Y | N | Drug Repurposing | Niclosamide | Efficacy - Viral load | Mild/Moderate Cases |
| CTRI/2020/ 05/024967 |
A Prospective, Open label observational clinical study to evaluate the efficacy and safety of MyVir tablets to improve immunity in quarantine patients of COVID-19 | India | N | N | Shelved drug or NME | MyVir | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 05/024959 |
A Multi-Centre, Open label, Two Arm Randomized, Pivotal Phase 2 Trial to Study the Efficacy and Safety of Itolizumab in COVID-19 Complications | India | Y | N | Drug Repurposing | Itolizumab | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 05/024962 |
Effectiveness of topical povidone iodine (PVP-I) oropharyngeal and intranasal application during the current coronavirus pandemic amongst COVID-19 positive patients in Andhra Pradesh, India | India | Y | N | Shelved drug or NME | Povidone-Iodine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| CTRI/2020/ 05/025013 |
Phase 2 Clinical Trial for the Evaluation of BCG as potential therapy for CoVID-I9 - COVID-19 BCG | India | Y | N | Drug Repurposing | BCG Vaccine | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 05/025022 |
An open label randomised controlled trial to assess the efficacy of Hydroxychloroquine in patients with mild COVID -19 illness with risk factors for severe disease. | India | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| CTRI/2020/ 05/025067 |
A randomized controlled trial of hydroxychloroquine prophylaxis for Healthcare Workers exposed to COVID-19 | India | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| CTRI/2020/ 05/025068 |
A Phase IIB open label randomized controlled trial to evaluate the efficacy and safety of Ivermectin in reducing viral loads in patients with hematological disorders who are admitted with COVID 19 infection - Not applicable | India | Y | N | Drug Repurposing | Ivermectin | Efficacy - Viral load | Other |
| CTRI/2020/ 05/025114 |
A Randomized, Open-label, multicenter study to evaluate the efficacy and safety of Favipiravir combined with STANDARD supportive care in adult Indian patients with mild to moderate COVID-19 | India | Y | N | Drug Repurposing | Favipiravir | Efficacy - Viral load | Mild/Moderate Cases |
| CTRI/2020/ 05/025167 |
Evaluation of Efficacy and Safety of Thymoquinone compared to Best supportive care in Patients with COVID-19. | India | Y | N | Shelved drug or NME | Thymoquinone | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 05/025209 |
AN OPEN LABEL RANDOMISED CONTROL TRIAL ON PASSIVE IMMUNIZATION WITH CONVALESCENT PLASMA IN SEVERE COVID-19 DISEASE - PICP19 | India | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| CTRI/2020/ 05/025224 |
Interventional study to assess the efficacy of Ivermectin with standard of care treatment versus standard of care in patients of COVID-19 at R D Gardi Medical College, Ujjain, India | India | Y | N | Drug Repurposing | Ivermectin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 05/025242 |
Population pharmacokinetics of hydroxychloroquine sulphate in healthcare workers given for prophylaxis against Corona Virus Disease 2019 (COVID 19) pandemic in India | India | N | N | Drug Repurposing | Hydroxychloroquine | Other | Exposed individuals (family members, HCP) |
| CTRI/2020/ 05/025271 |
A Randomized, Double-blind, Two arm, controlled clinical trial to compare the Efficacy and Safety of Mycobacterium w (Mw) administered along with Standard of care versus Placebo administered along with Standard of care, in adult, COVID 19 positive patients hospitalized but not critically ill. | India | Y | N | Shelved drug or NME | Mycobacterium | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| CTRI/2020/ 05/025277 |
A Randomized, Double-blind, Two arm, Placebo Controlled Clinical Trial to Evaluate the Efficacy and Safety of Mycobacterium w in preventing COVID-19 in subjects at risk of getting infected with COVID-19. | India | Y | N | Shelved drug or NME | Mycobacterium | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| CTRI/2020/ 05/025299 |
Convalescent Plasma to Limit Coronavirus Associated Complications: An Open label Clinical Study of Anti-SARS-CoV-2 Plasma in Hospitalized Patients with COVID-19 | India | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| CTRI/2020/ 05/025319 |
Angiotensin Receptor Blocker Losartan for prevention of COVID 19 complications: a randomized placebo controlled trial - LICCI | India | Y | N | Drug Repurposing | Losartan | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 05/025328 |
To Assess the Safety and Efficacy of Convalescent Plasma on outcome of COVID-19 Associated Complications - COVID PLASMA STUDY | India | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| CTRI/2020/ 05/025346 |
A Phase II, Open Label, Randomized Controlled Trial to Assess the Safety and Efficacy of Convalescent Plasma in Severe Covid-19 patients. | India | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| CTRI/2020/ 05/025369 |
A Multi-center, Randomized Controlled, Phase III Study to evaluate the Clinical Outcomes and Safety of Tocilizumab along with Standard of Care in Patients with Cytokine Release Syndrome associated with COVID-19 infection - None | India | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 06/025575 |
Safety and efficacy of antiviral combination therapy in symptomatic patients of Covid-19 infection - a randomised control trial - SEV-Covid Trial | India | Y | Y | Drug Repurposing | Oseltamivir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 06/025613 |
Melatonin For COVID 19 pre exposure prophylaxis in High Risk population . (MELATONIN IMMUNE BOOST COVID 19 STUDY ) | India | Y | N | Drug Repurposing | Melatonin | Efficacy - Infection rate (prevention) | Other |
| CTRI/2020/ 06/025704 |
A Phase 3, Prospective, Randomized, Open Label, Comparative, Clinical Study To Evaluate Efficacy And Safety Of Ulinastatin Plus Standard-Of-Care Compared To Standard-Of-Care In Treatment Of Acute Respiratory Distress Syndrome (ARDS) In Hospitalized COVID-19 Infection Patient | India | Y | N | Shelved drug or NME | Ulinastatin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 06/025760 |
Efficacy of Sofosbuvir in the management of hospitalized COVID 19 patients. | India | N | N | Drug Repurposing | Sofosbuvir | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 06/025799 |
A Randomized, Open Label, Prospective, Comparative, Parallel Group, Multicentre Study To Evaluate Efficacy And Safety Of Favipiravir With Supportive Care Versus Supportive Care Alone In Subjects With Mild To Moderate Coronavirus Disease (COVID-19). - NA | India | Y | N | Drug Repurposing | Favipiravir | Efficacy - Other efficacy | Mild/Moderate Cases |
| CTRI/2020/ 06/025803 |
Efficacy of Convalescent Plasma Therapy in Patients With COVID-19: A Randomized Control Trial | India | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - WHO scale | Severe Cases |
| CTRI/2020/ 06/025849 |
A Randomized, Open Label, 2-Treatment Groups Clinical Trial Evaluating the Safety and Efficacy of a Combination of Nitazoxanide and Hydroxychloroquine Versus Hydroxychloroquine Alone in the Acute Treatment of Moderate COVID-19 Patients | India | Y | N | Drug Repurposing | Nitazoxanide | Efficacy - Viral load | Mild/Moderate Cases |
| CTRI/2020/ 06/025854 |
Study to Evaluate the Effectiveness of BCG vaccine in Reducing Morbidity and Mortality in Elderly individuals in COVID-19 Hotspots in India - BCG for COVID-19 | India | N | N | Vaccine | Vaccine | Efficacy - WHO scale | Other |
| CTRI/2020/ 06/025928 |
Povidone Iodine Gargle and Intranasal application: A Quasi-Experimental study to reduce the spread of coronavirus infection in community with Covid-19 positive clusters. | India | N | N | Shelved drug or NME | Povidone-Iodine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| CTRI/2020/ 06/025957 |
A Randomized Open-Label Study To Evaluate The Efficacy And Safety Of Favipiravir And Umifenovir As Compared To Favipiravir Alone In Moderate Hospitalized Adult Indian COVID-19 Patients. | India | Y | N | Drug Repurposing | Umifenovir | Efficacy - WHO scale | Mild/Moderate Cases |
| CTRI/2020/ 06/026001 |
Randomised Controlled Trial of Ivermectin in hospitalised patients with COVID19 - RIVET-COV | India | Y | N | Drug Repurposing | Ivermectin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 06/026087 |
A phase II, randomized, controlled, open-label study to evaluate the efficacy and safety of Pegylated IFN alfa-2b in the treatment of adult patients diagnosed with SARS-CoV2 (COVID-19). | India | Y | N | Drug Repurposing | Interferon alpha-2b | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 06/026123 |
A phase II, Open label, randomized controlled trial to assess the safety and efficacy of convalescent plasma in severe COVID-19. - PLATINA TRIAL. | India | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - WHO scale | Severe Cases |
| CTRI/2020/ 06/026187 |
Phase II, open label, randomized controlled trial to evaluate Safety and Efficacy of ACT12 Tablet and ACT 13 dry syrup as an immunomodulator in adult Covid 19 positive patients - Nil | India | Y | N | Shelved drug or NME | ACT12, ACT13 | Efficacy - Other efficacy | Mild/Moderate Cases |
| CTRI/2020/ 06/026189 |
Randomized, Double Blind, Parallel Group Study of Vitamin D3 & Magnesium in Covid 19 Infection - Covid | India | Y | N | Drug Repurposing | Vitamin D, Magnesium | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| CTRI/2020/ 06/026191 |
Randomized, Double Blind, Comparative, Parallel group study of Vitamin D3 ( Cholecalciferol ) Vitamin K2-7 & magnesium in prophylaxis of COVID-19 infection in health care professionals - Covid19 | India | Y | N | Drug Repurposing | Vitamin K, Magnesium | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| CTRI/2020/ 06/026192 |
A randomised, double-blind, placebo-controlled, phase 2 trial investigating the safety and efficacy of C21 in hospitalised subjects with COVID-19 infection not requiring mechanical ventilation - The ATTRACT Trial | India | Y | N | Shelved drug or NME | C21 | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 06/026193 |
A randomized control study to evaluate the therapeutic effects of Lithium in Covid-19 patients | India | Y | N | Drug Repurposing | Lithium | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 06/026222 |
A Phase II Safety and Efficacy Study on prognosis of Moderate Pneumonia in COVID-19 patients with Regular Intravenous Immunoglobulin Therapy | India | Y | N | Cell/Blood based | Immunoglobulin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| CTRI/2020/ 06/026220 |
An Open Label, Randomized, Multicenter, Controlled Clinical Study to Evaluate the Efficacy and Safety of Nafamostat Mesilate in the Treatment of Moderate COVID-19 Disease | India | Y | N | Drug Repurposing | Nafamostat | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| CTRI/2020/ 06/026232 |
A Clinical Trial to Study the Efficacy of â??Ivermectinâ?? in the prevention of Covid-19. .A Single Arm Study. â?? | India | N | N | Drug Repurposing | Ivermectin | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| CTRI/2020/ 07/026340 |
Prospective study to assess therapeutic role of Zinc in COVID-19 patients | India | Y | N | Drug Repurposing | Zinc | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| CTRI/2020/ 07/026352 |
A prospective, randomized, adaptive, phase I/II clinical study to evaluate the safety and immunogenicity of Novel Corona Virus -2019-nCov vaccine candidate of M/s Cadila Healthcare Limited by intradermal route in healthy subjects | India | Y | N | Vaccine | Safety | Other | |
| ACTRN12620 000731998 |
Safety and efficacy of a pharmacological strategy using Losartan in hospital patients with COVID-19 | Australia | N | N | Drug Repurposing | Losartan | Safety | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 034796 |
the efficacy and safety of heparin in the treatment of novel coronavirus pneumonia (COVID-19): a prospective, randomized, controlled trial | China | N | N | Drug Repurposing | LMWH | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| ChiCTR2000 034780 |
Randomized, Double Blind, Parallel Placebo Controlled, Phase III Clinical Trial to Evaluate the Safety and Protective Efficacy of Inactivated SARS-CoV-2 Vaccine in Healthy Population Aged 18 Years and above | United Arab Emirates | Y | Y | Vaccine | ND:Vero cell vaccine | Efficacy - Infection rate (prevention) | General population |
| ChiCTR2000 034825 |
Safety and Immunogenicity of SARS-CoV-2 mRNA Vaccine (BNT162b1) in Chinese Healthy Subjects: A Phase I, Randomized, Placebo-controlled, Observer-blind Study | China | N | N | Shelved drug or NME | BNT162b1 | Safety | Other |
| ACTRN12620 000588998 |
The safety and efficacy of STC3141 in patient with COVID-19 ARDS require intensive care | Australia | Y | N | Shelved drug or NME | STC3141 | Efficacy - Oxygen parameters | Severe Cases |
| ACTRN12620 000566932 |
BEAT COVID-19: A Bayesian adaptive randomised controlled trial platform to evaluate the efficacy of interventions for high risk older patients with COVID-19 in reducing the risk of hospital admission or death. | Australia | Y | N | Drug Repurposing | Efficacy - Other efficacy | Mild/Moderate Cases | |
| ACTRN12620 000674932 |
A Phase 1 Randomised, Double-Blind, Placebo-Controlled, Dosage-Escalation, Single Centre Study To Evaluate The Safety And Immunogenicity Of An Adjuvanted SARS-CoV-2 Sclamp Protein Subunit Vaccine (COVID-19 vaccine) In Healthy Adults Aged 18 To 55 Years Old | Australia | Y | N | Shelved drug or NME | SARS-CoV-2 Sclamp Protein Subunit Vaccine | Safety | Other |
| ISRCTN1432 6006 |
Protecting frontline health care workers from COVID-19 with hydroxychloroquine pre-exposure prophylaxis: a randomized, placebo-controlled multi-site trial in Toronto, Canada | Canada | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| ISRCTN4873 4830 |
RAAS blockagE in SARS COV-2 Critically ill patiEnts– a Randomized controlled trial | Sweden | Y | N | Drug Repurposing | Losartan | Efficacy - Other efficacy | Severe Cases |
| ISRCTN8995 1424 |
A phase III randomized controlled trial to determine safety, efficacy, and immunogenicity of the non-replicating ChAdOx1 nCoV-19 vaccine | United Kingdom | Y | N | Vaccine | ND:ChAdOx1 vaccine | Efficacy - Infection rate (prevention) | Other |
| ISRCTN5904 8638 |
Sulodexide in the treatment of early stages of COVID-19 | Mexico | Y | N | Drug Repurposing | Sulodexide | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| ISRCTN1707 2692 |
A first-in-human clinical trial to assess the safety and immunogenicity of a self-amplifying ribonucleic acid (saRNA) vaccine encoding the S glycoprotein of SARS-CoV-2, the causative agent of COVID-19 | United Kingdom | Y | N | Vaccine | ND:Vaccine | Safety | General population |
| ISRCTN8397 1151 |
An international randomized trial of additional treatments for COVID-19 in hospitalized patients who are all receiving the local standard of care | Switzerland | Y | Y | Drug Repurposing | Remdesivir, Chloroquine, Hydroxychloroquine, Lopinavir/ritonavir, Interferon beta | Efficacy - WHO scale | General population, Severe Cases |
| NL8523 | Volatile agents for sedation in patients with COVID-19-associated acute respiratory distress syndrome – a pilot study | Netherlands | N | N | Drug Repurposing | Isoflurane | Efficacy - Oxygen parameters | Severe Cases |
| NL8578 | Alkaline phosphatase for reducing systemic inflammatory response syndrome (SIRS) in patients with Sars-CoV-2 infection and acute respiratory insufficiency (COVID 19) | Netherlands | N | N | Drug Repurposing | Alkaline phosphatase | Efficacy - Oxygen parameters | Severe Cases |
| NL8609 | Enhancing the BCG-induced trained immunity response by addition of bisphosphonate or MMR vaccine: a possible preventive approach against COVID-19 (BCG-PLUS) | Netherlands | Y | N | Drug Repurposing, Vaccine | ND:BCG vaccine, Bisphosphonate, ND:MMR vaccine | Efficacy - Other efficacy | General population |
| NL8633 | Convalescent plasma compared to standard plasma for treatment of hospitalized non-ICU patients with COVID-19 infections | Netherlands | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NL8726 | Randomized, placebo-controlled multiple dose study to evaluate the effect of hydroxychloroquine on the general immuno-competence in young and elderly healthy male volunteers | Netherlands | N | N | Drug Repurposing | Hydroxychloroquine | Other | Other |
| NL8490 | An open label randomized controlled trial of chloroquine, hydroxychloroquine or only supportive care in patients admitted with moderate to severe COVID-19 | Netherlands | Y | Y | Drug Repurposing | Chloroquine, Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| DRKS000215 73 |
Impact of pantoprazole on absorption and disposition of hydroxychloroquine, a drug used in Covid-19 - K724_HCQ-PPI | Germany | Y | N | Drug Repurposing | Pantoprazole | Other | General population |
| DRKS000212 14 |
Improvement of the nutritional status regarding nicotinamide (vitamin B3) and the course of COVID-19 disease - COVit | Germany | Y | N | Drug Repurposing | Nicotinamide | Efficacy - Other efficacy | Mild/Moderate Cases |
| DRKS000222 03 |
Efficacy & Safety of Sofosbuvir/Daclatasvir treatment in COVID-19: A randomized, controlled study | Egypt | Y | Y | Drug Repurposing | Sofosbuvir, Daclatasvir, Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| RPCEC00000 306 |
Randomized control clinical trial of CIGB 2020 in contacts and SARS-CoV-2 infection suspects (COVID-19) | Cuba | Y | N | Shelved drug or NME | CIGB 2020 vaccine | Efficacy - Other efficacy | Exposed individuals (family members, HCP) |
| RPCEC00000 307 |
Evaluation of the effect and safety of HeberFERON versus Heberon alfa in patients infected with the SARS-CoV-2 coronavirus (COVID-19). | Cuba | Y | N | Drug Repurposing | Interferon Alpha Gamma | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| RPCEC00000 308 |
Pharmacodynamics and safety of recombinant interferon alfa 2b, by different routes of administration, in healthy volunteers (COVID-19). | Cuba | Y | Y | Drug Repurposing | Interferon alpha-2b | Other | General population |
| RPCEC00000 309 |
Evaluation of the effect of 0.002% sodium chloride saline (activated) administered intravenously and / or mists in patients with covid-19. (COVID-19) - TX-COVID19 | Mexico | Y | N | Drug Repurposing | Sodium chloride | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| RPCEC00000 311 |
Treatment of patients with severe SARS-CoV-2 pneumonia with the anti-CD6 monoclonal antibody itolizumab. (COVID-19) - VICTORIA | Cuba | N | N | Drug Repurposing | Itolizumab | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| RPCEC00000 313 |
Administration of the CIGB-258 peptide in patients with COVID-19 under intensive therapy regimen (COVID-19) | Cuba | N | N | Shelved drug or NME | CIGB-258 | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| RPCEC00000 314 |
Population impact of intervention based on the administration of a single dose of the Outer Membrane Vesicle (OMV) complex contained in VA-MENGOC-BC to stimulate the innate response against SARS-CoV-2 (COVID-19) - VME-CORONA | Cuba | N | N | Vaccine | ND:Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| RPCEC00000 315 |
Evaluation of the synergistic effect of sequential administration of two immunostimulant products: Immuno-1 and Immuno-2 for the stimulation of the immune response in older adults. Exploratory study (COVID-19) - InmunoCorona | Cuba | Y | N | Shelved drug or NME | Immuno-1, Immuno-2 | Other | Other |
| RPCEC00000 316 |
Evaluation of the effect and safety of BIOMODULIN T(R) for the stimulation of the immune response in patients with Stage 5 Chronic Kidney Disease under an iterated hemodialysis regimen. Exploratory study. (COVID-19) | Cuba | Y | N | Shelved drug or NME | Biomodulin T | Efficacy - Other efficacy | Other |
| RPCEC00000 317 |
Application of the CIGB 300 as Antiviral in the treatment of patients with a diagnosis of Covid 19. (COVID-19) | Cuba | Y | N | Shelved drug or NME | CIGB 300 | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| RPCEC00000 321 |
Expanded clinical use of CIGB-258 in the treatment of serious or critically ill patients by COVID-19 (COVID-19) | Cuba | N | N | Shelved drug or NME | CIGB-258 | Efficacy - Oxygen parameters | Severe Cases |
| JPRN-jRCTs 031190269 |
A multicenter, open-label, randomized controlled phase II study to evaluate the efficacy and safety of inhaled ciclesonide for asymptomatic and mild patients with COVID-19 - RACCO trial | Japan | Y | N | Drug Repurposing | Ciclesonide | Efficacy - Other efficacy | Mild/Moderate Cases |
| 2020-00178 3-28 |
REDUCING ABSENCE FROM WORK OF HEALTHCARE WORKERS DUE TO COVID-19 INFECTION BY BCG (BACILLUS CALMETTE-GUÉRIN) VACCINATION | Hungary | Y | N | Vaccine | BCG vaccine | Efficacy - Other efficacy | Exposed individuals (family members, HCP) |
| 2020-00145 9-42 |
Ruxolitinib Treatment in Patients with Severe COVID-19 Infection. A Danish Safety and Efficacy Study. | Denmark | N | N | Drug Repurposing | Ruxolitinib | Efficacy - Mortality | Severe Cases |
| 2020-00119 4-69 |
Pilot study to evaluate the efficacy and safety of mefloquine as prophylaxis in people exposed to the disease caused by the SARS-CoV-2 coronavirus (COVID-19) | Spain | Y | N | Drug Repurposing | Mefloquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| 2020-00212 3-11 |
RANDOMIZED CLINICAL TRIAL OF PARALLEL GROUPS TO COMPARE THE EFFICACY OF HYDROXYCHLOROQUINE VS. CYCLOSPORINE - HYDROXYCHLOROQUINE IN THE TREATMENT OF COVID-19 PNEUMONIA. | Spain | Y | N | Drug Repurposing | Cyclosporine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| 2020-00167 8-31 |
Randomized controlled trial evaluating the efficacy of vaccination with Bacillus Calmette and Guérin (BCG) in the Prevention of COVID-19 via the strengthening of innate immunity in Health Care Workers.” COVID-BCG | France | Y | N | Vaccine | BCG Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| 2020-00152 7-14 |
COVID-19: addition of azithromycin to chloroquine treatment | Netherlands | Y | N | Drug Repurposing | Azithromycin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| 2020-00188 8-90 |
Using BCG vaccine to enhance non-specific protection of health care workers during the COVID-19 pandemic. A randomised controlled multi-center trial. | Denmark | Y | N | Vaccine | BCG vaccine | Efficacy - Other efficacy | Exposed individuals (family members, HCP) |
| 2020-00142 1-31 |
Clinical trial randomized, unblinded and controled for evaluation of efficacy and safety of hydroxychloroquine chemoprophylaxis against SARS-CoV-2 (COVID-19) infection in healthcare professionals. | Spain | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| 2020-00154 6-19 |
A First-in-Human Study in Adult Subjects Hospitalized with Covid-19 to Investigate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, Immunogenicity and Efficacy of ARGX-117 | Belgium | N | N | Shelved drug or NME | ARGX-117 | Safety | Mild/Moderate Cases, Severe Cases |
| 2020-00202 7-10 |
A prospective, multicenter, randomized PHASE II clinical trial of enzalutamide treatment to decrease the morbidity in patients with Corona virus disease 2019 (COVID-19) | Sweden | Y | N | Drug Repurposing | Enzalutamide | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| 2020-00169 7-30 |
PRE-EXPOSURE PROPHYLAXIS WITH HYDROXYCHLOROQUINE IN SANITARIES HIGHLY EXPOSED TO COVID-19. (COVIDNA) | Spain | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Other efficacy | Exposed individuals (family members, HCP) |
| 2020-00168 6-36 |
A double-blind, randomized study versus placebo of avdoralimab (IPH5401), an anti-C5aR antibody, in patients with COVID-19 induced pneumonia | France | Y | N | Shelved drug or NME | Avdoralimab | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| 2020-00141 7-21 |
An open label single center randomized controlled trial to evaluate the effect of hydroxychloroquine on viral shedding in mild COVID-19 | Belgium | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases |
| 2020-00144 0-26 |
Pilot, double-blind clinical trial to evaluate the efficacy and safety of pre-exposure use of hydroxychloroquine versus placebo in the prevention of SARS-CoV-2 (COVID-19) infection in healthcare personnel. | Spain | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| 2020-00198 7-28 |
PRECOV: a randomized controlled clinical trial on the effects of hydroxychloroquine in the prevention of COVID-19 in healthcare workers at risk | Italy | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| 2020-00244 9-41 |
Pilot trial on early treatment with hydroxychloroquine in patients with COVID-19 who do not have hospital admission at diagnosis. | Spain | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| 2020-00132 7-13 |
A SINGLE-BLINDED RANDOMIZED, PLACEBO-CONTROLLED PHASE II TRIAL OF PROPHYLACTIC TREATMENT WITH ORAL AZITHROMYCIN VERSUS PLACEBO IN CANCER PATIENTS UNDERGOING ANTINEOPLASTIC TREATMENT DURING THE COVID-19 PANDEMIC | Austria | N | N | Drug Repurposing | Azithromycin | Efficacy - Infection rate (prevention) | Other |
| 2020-00199 4-66 |
Ramdomised clinical trial of ivermectin for treatment and prophylaxis of COVID-19 | Spain | Y | N | Drug Repurposing | Ivermectin | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP), Mild/Moderate Cases |
| 2020-00223 3-15 |
A randomized, single blind, placebo-controlled, parallel-arm study to investigate the safety and preliminary efficacy of Niclosamide and Camostat alone and in combination to treat Covid-19 (“NICCAM”) | Germany | Y | Y | Drug Repurposing | Niclosamide, Camostat | Safety | Mild/Moderate Cases |
| 2020-00177 7-71 |
Ruxolitinib therapy to Avoid Ventilation and improve outcome for deteriorating COVID-19 patiENts - RAVEN | United Kingdom | N | N | Drug Repurposing | Ruxolitinib | Efficacy - Other efficacy | Severe Cases |
| 2020-00174 0-26 |
A multi-centre open-label two-arm randomised superiority clinical trial of Azithromycin versus usual care In Ambulatory COVID-19 (ATOMIC2) | United Kingdom | Y | N | Drug Repurposing | Azithromycin | Efficacy - Other efficacy | Mild/Moderate Cases |
| 2020-00180 3-17 |
A Phase 2/3 Single-Arm, Open-Label Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Remdesivir (GS-5734™) in Participants from Birth to < 18 Years of Age with COVID-19 | United Kingdom | N | N | Shelved drug or NME | Remdesivir | Safety | Mild/Moderate Cases, Severe Cases, Other |
| 2020-00161 8-39 |
A Phase II, randomized, open–label study to evaluate the efficacy and tolerability of treatment with vafidemstat in combination with standard of care treatment to prevent Acute Respiratory Distress Syndrome (ARDS) in adult severely ill patients with COVID-19. | Spain | Y | N | Drug Repurposing | Vafidemstat | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| 2020-00209 1-12 |
Multicenter, randomized, double-blind, placebo-controlled study investigating efficacy, safety and tolerability of ivermectin HUVE-19 in patients with proven SARS-CoV-2 infection (COVID-19) and manifested clinical symptoms. | Bulgaria | Y | N | Drug Repurposing | Ivermectin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| 2020-00221 1-21 |
A Phase II, randomised, double-blind, placebo-controlled clinical trial to assess the safety and efficacy of AZD1656 in diabetic patients hospitalised with suspected or confirmed COVID-19. The ARCADIA Trial | United Kingdom | Y | N | Shelved drug or NME | AZD1656 | Efficacy - WHO scale | Other |
| 2020-00144 8-24 |
Preventing Pulmonary Complications in Surgical Patients at Risk of COVID-19 | United Kingdom | Y | Y | Drug Repurposing | Hydroxychloroquine, Ritonavir/lopinavir | Efficacy - Other efficacy | General population |
| 2020-00175 0-22 |
MATIS: Phase 2/3, Randomised, Open-Label, Single-Site, Multi-Arm Trial of Ruxolitinib Plus Best Supportive Treatment (BST) versus Fostamatinib Plus BST versus BST for COVID-19 pneumonia | United Kingdom | Y | Y | Drug Repurposing | Ruxolitinib, Fostamatinib | Efficacy - Other efficacy | Mild/Moderate Cases |
| 2020-00172 1-31 |
Can a sinus rinse and mouth wash reduce viral load in COVID-19 positive individuals? | United Kingdom | Y | N | Shelved drug or NME | iodonated Povidone | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| 2020-00129 6-33 |
Efficacy and safety of 72-hour infusion of Prostacyclin (1 ng/kg/min) in patients with COVID-19 induced respiratory failure – a multicentre randomized, placebo-controlled, blinded, investigator-initiated trial | Denmark | Y | N | Drug Repurposing | Iloprost | Efficacy - Oxygen parameters | Severe Cases |
| 2020-00167 1-32 |
A multi-center, open-label, randomized parallel controlled evaluation on the efficacy and safety of BDB-001 injection in the treatment of progressive severe COVID-19 in phase II | Spain | Y | N | Shelved drug or NME | BDB-001 | Efficacy - Oxygen parameters | Severe Cases |
| 2020-00160 5-23 |
Effectiveness of the combined treatment with hydroxycloroquine and azithromycin vs lopinavir/ritonavir + hydroxycloroquine in hospitalized patients with confirmed COVID-19 infection | Spain | Y | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| 2020-00247 2-12 |
Lanadelumab for treatment of COVID-19 disease | Netherlands | Y | N | Drug Repurposing | Lanadelumab | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| 2020-00192 1-30 |
Steroids and unfractionated heparin in critically-ill patients with pneumonia from COVID-19 infection. A multicenter, interventional, randomized, three arms study design. | Italy | Y | N | Drug Repurposing | Methylprednisolone | Efficacy - Mortality | Severe Cases |
| 2020-00192 9-31 |
Controlled, randomized, non-blind trial on the usefulness of pioglitazone treatment in patients with type 2 diabetes mellitus and COVID-19. | Spain | Y | N | Drug Repurposing | Pioglitazone | Efficacy - Clinical improvement (except WHO scale) | Other |
| 2020-00130 8-40 |
Intermediate dose enoxaparin in hospitalized patients with moderate-severe COVID-19: a pilot phase II single-arm study, INHIXACOVID19 | Italy | N | N | Drug Repurposing | Enoxaparin | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| 2020-00158 7-29 |
Hydroxychloroquine efficacy in preventing SARS-CoV-2 infection and CoVid-19 disease severity during pregnancy | Spain | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Exposed individuals (family members, HCP), Mild/Moderate Cases |
| 2020-00199 5-13 |
A multicentre, open-label clinical trial to evaluate the effectiveness and safety of intravenous tocilizumab for treating patients with COVID-19 pneumonia: the BREATH-19 Study | Spain | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| 2020-00212 0-37 |
An open-label, adaptive randomized, controlled multicenter study to evaluate the efficacy and safety of RESP301 plus standard of care (SOC) compared to SOC alone in hospitalized participants with COVID-19 requiring supplemental oxygen (NOCoV2) | United Kingdom | Y | N | Shelved drug or NME | RESP301 | Efficacy - WHO scale | Severe Cases |
| 2020-00190 4-41 |
Glasgow Early Treatment Arm FavIpiravir? : A randomized controlled study of favipiravir as an early treatment arm in COVID-19 patients | United Kingdom | Y | N | Drug Repurposing | Favipiravir | Efficacy - WHO scale | Mild/Moderate Cases |
| 2020-00176 5-37 |
Pragmatic clinical trial to evaluate the efficacy of hydroxychloroquine in the treatment of COVID-19 infection in two cohorts: patients with oncohaematological disease and SARS-CoV-2 positive without radiological alteration and sars-cov-2 positive professionals without radiological alteration | Spain | N | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Other |
| 2020-00163 5-27 |
A Phase 2, Randomized, Open-label Clinical Trial comparing the Safety and Efficacy of Hydroxychloroquine, and Standard of Care to a Combination of Masitinib, Isoquercetin, and Standard of Care for the Treatment of Hospitalized Moderate and Severe COVID-19 Patients. | France | Y | N | Drug Repurposing | Masitinib, Isoquercetin | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| 2020-00153 6-98 |
Prophylaxis of COVID-19 infection with hydroxychloroquine in healthcare personnel with high risk of infection. | Spain | N | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| 2020-00127 0-29 |
An adaptive Phase 2/3, randomized, open-label study assessing efficacy and safety of hydroxychloroquine for hospitalized patients with moderate to severe COVID-19 | United Kingdom | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| 2020-00138 6-37 |
Uno studio randomizzato multicentrico in aperto per valutare l’efficacia della somministrazione precoce del Tocilizumab (TCZ) in pazienti affetti da polmonite da COVID-19. | Italy | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| 2020-00160 3-16 |
A RANDOMIZATION, MULTICENTRIC, OPEN-LABEL, CONTROLLED, CLINICAL TRIAL TO INVESTIGATE THE EFFECTIVENESS OF EARLY CHOLCHICINE ADMINISTRATION IN PATIENTS OVER 70 YEARS OF AGE WITH HIGH RISK OF DEVELOPING SEVERE PULMONARY COMPLICATIONS ASSOCIATED WITH CORONAVIRUS SARS-CoV2 PNEUMONIA (COVID-19) | Spain | Y | N | Drug Repurposing | Colchicine | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| 2020-00150 2-38 |
A randomised, double blind, placebo controlled, Phase 2 trial investigating the safety and efficacy of C21 in hospitalised subjects with COVID-19 infection not requiring mechanical ventilation | United Kingdom | Y | N | Shelved drug or NME | C21 | Other | Mild/Moderate Cases |
| 2020-00170 4-42 |
Controlled and randomised trial to assess the safety and efficacy of hydroxychloroquine chemoprophylaxis in SARS CoV2 infection in hospital healthcare personnel (Sanitarios sin COVID-19 -SANsinCOVID-19). | Spain | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| 2020-00184 1-38 |
Colchicine for the Treatment of Hyperinflammation associated with Pneumonia due to COVID-19 | Spain | Y | N | Drug Repurposing | Colchicine | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| 2020-00149 2-33 |
Interest in the administration of Dornase alpha aerosol in ARDS secondary to respiratory infection by the coronavirus SRASCoV-2 / COVID-19 | France | Y | N | Drug Repurposing | Dornase alfa | Efficacy - Oxygen parameters | Severe Cases |
| 2020-00176 6-11 |
Losartan and spironolactone treatment for COVID-19 patients with acute respiratory failure in intensive care unit | France | Y | N | Drug Repurposing | Losartan, Spironolactone | Efficacy - Other efficacy | Severe Cases |
| 2020-00180 8-42 |
PHASE II CLINICAL TRIAL, SINGLE-BLIND, RANDOMIZED, PLACEBO CONTROLLED TO EXPLORE THE EFFECTIVENESS AND SAFETY OF MELATONIN I.V. IN PATIENTS WITH COVID-19 ENTERED INTO THE ICU (MELCOVID STUDY) | Spain | Y | N | Drug Repurposing | Melatonin | Efficacy - Mortality | Severe Cases |
| 2020-00120 9-22 |
Platform Randomised trial of INterventions against COVID-19 In older peoPLE | United Kingdom | Y | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Other efficacy | General population |
| 2020-00144 5-39 |
PRAGMATIC, CONTROLLED, OPEN, SINGLE CENTER, RANDOMIZED, PHASE II CLINICAL TRIAL TO EVALUATE METHYLPREDNISOLONE PULSES AND TACROLIMUS IN HOSPITALIZED PATIENTS WITH SEVERE PNEUMONIA SECONDARY TO COVID-19 (TACROVID) | Spain | Y | N | Drug Repurposing | Methylprednisolone, Tacrolimus | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| 2020-00180 7-18 |
A Phase 2, Randomized, Double-Blind, Placebo-Controlled, Parallel-group, Multi-center Study of an Inhaled Pan-Janus Kinase Inhibitor, TD-0903, to Treat Symptomatic Acute Lung Injury Associated with COVID-19 | United Kingdom | Y | N | Shelved drug or NME | TD-0903 | Safety | Severe Cases |
| 2020-00168 2-36 |
Double-blind, placebo-controlled phase I/II clinical trial to evaluate the safety and efficacy of allogeneic mesenchymal stem cells (MSV®-allo) in acute respiratory failure in patients with COVID-19 pneumonia. | Spain | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Other efficacy | Severe Cases |
| 2020-00136 7-88 |
Efficacy and safety of novel treatment options for adults with COVID-19 pneumonia. A double-blinded, randomized, multi-stage, 6-armed placebo-controlled trial in the framework of an adaptive trial platform | Denmark | Y | Y | Drug Repurposing, Cell/Blood based | Hydroxychloroquine, Baricitinib, Sarilumab, ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| 2020-00203 2-69 |
Single-center, randomized, open-label clinical trial on the efficacy of tocilizumab in modifying the inflammatory parameters of patients with COVID-19 | Spain | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Other efficacy | Mild/Moderate Cases |
| 2020-00164 0-26 |
A pilot, open label, phase II clinical trial of nebulised recombinant tissue-Plasminogen Activator (rtPA)in patients with COVID-19 ARDS: The Plasminogen Activator COVID-19 ARDS (PACA) trial | United Kingdom | N | N | Drug Repurposing | Alteplase | Efficacy - Oxygen parameters | Severe Cases |
| 2020-00211 1-22 |
A multi-centre, randomised, double-blind, placebo-controlled phase III study assessing the impact of BCG vaccination on the incidence and course of SARS-CoV-2 infection among healthcare workers in Poland during the COVID-19 pandemic. | Poland | Y | N | Vaccine | ND:Vaccine | Efficacy - Mortality | Exposed individuals (family members, HCP) |
| 2020-00218 6-34 |
Efficacy of the early use of corticotherapy in CoV-2 infection to prevent the progression of acute respiratory distress syndrome (ARDS) in COVID-19 | Spain | N | N | Drug Repurposing | Methylprednisolone | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| 2020-00247 6-13 |
A study to assess the safety and efficacy of Clazakizumab in patients with COVID-19 and kidney disease. | United Kingdom | N | N | Shelved drug or NME | Clazakizumab | Efficacy - Other efficacy | Other |
| 2020-00146 9-35 |
A multicenter, open, randomized, non-commercial study to evaluate the efficacy and safety of chloroquine phosphate in the outpatient treatment of COVID-19 in combination with telemedicine care for patients with SARS-CoV-2 infection with the risk of developing complications to reduce the risk of COVID-19-related hospitalization and death. | Poland | Y | N | Drug Repurposing | Chloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| 2020-00180 5-21 |
Prospective, Randomized, Parallel-Group, Open-Label Study to Evaluate the Efficacy and Safety of IMU-838, in Combination with Oseltamivir, in Adults with Coronavirus Disease COVID-19 | United Kingdom | Y | N | Shelved drug or NME | IMU-838 | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| 2020-00125 0-21 |
COVID-19 - Epidemiology of SARS-CoV-2 and Mortality to Covid19 Disease upon Hydroxychloroquine and Azithromycin Therapy in French Cancer patients | France | N | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Clinical improvement (except WHO scale) | Other |
| 2020-00143 7-12 |
Random, controlled, open, one-site clinical trial in adult patients with COVID-19 severe pneumonia treated with immunomodulatory drugs | Spain | Y | Y | Drug Repurposing | Cyclosporine, Tocilizumab | Efficacy - Mortality | Severe Cases |
| 2020-00165 9-42 |
Pilot study of antithrombin as prophylaxis of acute respiratory distress syndrome in patients with COVID-19 | Spain | Y | N | Cell/Blood based | ND:Human antithrombin | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| 2020-00188 2-36 |
ANTI-INFLAMMATORY CLARITHROMYCIN TO IMPROVE SARS-CoV-2 (COVID-19) INFECTION EARLY: THE ACHIEVE OPEN-LABEL NON-RANDOMIZED CLINICAL TRIAL | Greece | N | N | Drug Repurposing | Clarithromycin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| 2020-00147 4-29 |
Pilot study to evaluate the potential of ivermectin to reduce COVID-19 transmission | Spain | Y | N | Drug Repurposing | Ivermectin | Efficacy - Viral load | Mild/Moderate Cases |
| 2020-00164 3-13 |
A randomised double-blind placebo-controlled trial of Brensocatib (INS1007) in patients with severe COVID-19 | United Kingdom | Y | N | Shelved drug or NME | Brensocatib | Efficacy - WHO scale | Severe Cases |
| 2020-00182 5-29 |
CLINICAL TRIAL OF THE USE OF ANAKINRA (ANTI IL-1) IN CYTOKINE STORM SYNDROME (CSS) SECONDARY TO COVID-19 | Spain | Y | N | Drug Repurposing | Anakinra | Efficacy - Oxygen parameters | Severe Cases |
| 2020-00160 6-33 |
Randomized clinical trial to evaluate the efficacy of hydroxychloroquine associated or not with azithromycin as a treatment for COVID-19 infection. | Spain | Y | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Viral load | Mild/Moderate Cases |
| 2020-00124 3-15 |
Covid-19: A randomized, open-label, adaptive, proof-of- concept clinical trial of new antiviral drug candidates against SARS-CoV-2. | Belgium | Y | N | Drug Repurposing | Itraconazole | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| 2020-00137 5-32 |
Pre-emptive tocilizumab in hypoxic COVID-19 patients, a prospective randomized trial | Netherlands | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| 2020-00189 0-56 |
Double-blind randomized placebo-controlled clinical trial to evaluate the efficacy and safety of the use of intravenous gammaglobulins in the treatment of patients with COVID-19 | Spain | Y | N | Cell/Blood based | ND:Gamma globulin | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| 2020-00123 6-10 |
COUNTER-COVID - Oral imatinib to prevent pulmonary vascular leak in COVID-19 – a randomized, single-blind, placebo controlled, clinical trial in patients with severe COVID-19 disease | Netherlands | Y | N | Drug Repurposing | Imatinib | Efficacy - Other efficacy | Severe Cases |
| 2020-00182 3-15 |
Evaluation of the concentration-effect relationship of enoxaparin for thromboembolic prevention in COVID-19 resuscitation patients. COV-ENOX study | France | N | N | Drug Repurposing | Enoxaparin | Efficacy - Other efficacy | Severe Cases |
| 2020-00195 3-36 |
A Multicenter, Randomized, Open-label, Parallel Group Pilot Study to Evaluate the Safety and Efficacy of Prolastin® plus Standard Medical Treatment (SMT) versus SMT alone in Hospitalized Subjects with COVID-19. | Spain | Y | N | Drug Repurposing | Prolastin | Efficacy - Other efficacy | Severe Cases |
| 2020-00169 6-32 |
A Multicenter, Randomized, Open-label Parallel Group Pilot Study to Evaluate Safety and Efficacy of High Dose Intravenous Immune Globulin (IVIG) plus Standard Medical Treatment (SMT) versus SMT alone in Hospitalized Subjects with COVID-19 | Spain | Y | N | Cell/Blood based | ND:Human normal immunoglobulin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| 2020-00149 3-29 |
Charité trial of Cenicriviroc (CVC) treatment for COVID-19 patients | Germany | Y | N | Shelved drug or NME | Cenicriviroc | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| 2020-00227 4-28 |
Usefulness of vitamin D on morbidity and mortality of SARS-COV-2 virus infection (Covid-19) at the Central University Hospital of Asturias | Spain | Y | N | Drug Repurposing | Cholecalciferol | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| 2020-00196 0-28 |
Efficacy of vitamin D treatment in patients diagnosed with pneumonia who require hospital admission and have vitamin D deficiency and a positive diagnosis for SARS-Cov-2 (COVID-19) | Spain | Y | N | Drug Repurposing | Vitamin D3 | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| 2020-00184 9-39 |
Aerosolized DNase I for the treatment of severe respiratory failure in COVID-19 - a phase 2, randomized controlled trial | Sweden | Y | N | Drug Repurposing | Dornase alfa | Efficacy - Oxygen parameters | Severe Cases |
| 2020-00193 4-37 |
USE OF CORTICOSTEROIDS IN PATIENTS WITH SARS-COV2 CORONAVIRUS INFECTION (GLUCOCOVID). Pragmatic trial inserted in real practice during a pandemic COVID-19 | Spain | Y | N | Drug Repurposing | Methylprednisolone | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| 2020-00229 3-28 |
Resolving inflammatory storm in COVID-19 patients by Omega-3 polyunsaturated fatty acids. - A single-blind, randomized, placebo-controlled feasibility study | Sweden | Y | N | Drug Repurposing | Omegaven | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| 2020-00155 8-23 |
Hydroxychloroquine sulfate early administration in symptomatic out of hospital COVID-19 positive patients. Hydro-Stop-COVID19 Trial | Italy | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases |
| 2020-00245 6-21 |
Enhancing the BCG-induced trained immunity response by addition of bisphosphonate or MMR vaccine: a possible preventive approach against COVID-19 (BCG-PLUS) | Netherlands | Y | Y | Drug Repurposing, Vaccine | MMR Vaccine, Alendronic acid | Other | General population |
| 2020-00174 7-21 |
Impact of Montelukast as add on treatment to the novel coronavirus pneumonia (COVID-19): an investigator-initiated open labelled randomized controlled pragmatic trial | Portugal | Y | N | Drug Repurposing | Montelukast | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| 2020-00216 6-13 |
Randomized, open, multicenter phase II clinical trial, proof of concept, to evaluate efficacy and safety of Icatibant in hospitalized patients with SARS-COV-2 (COVID-19) without assisted ventilation compared with standard care | Spain | Y | N | Drug Repurposing | Icatibant | Safety | Mild/Moderate Cases, Severe Cases |
| 2020-00231 0-41 |
Intravenous Metoprolol in Respiratory Distress Due to COVID-19: Pilot Study "MADRID-COVID" | Spain | N | N | Drug Repurposing | Metoprolol | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| 2020-00177 0-30 |
COVID 19: Experimental use of tocilizumab (Roactemra®) in severe SARS-CoV-2 related pneumonia. | Belgium | Y | N | Drug Repurposing | Tocilizumab | Efficacy - WHO scale | Severe Cases |
| 2020-00133 3-13 |
Dexamethasone associated with hydroxychloroquine vs. hydroxychloroquine alone for the early treatment of severe ARDS caused by COVID-19 : a randomized controlled trial | France | Y | N | Drug Repurposing | Dexamethasone | Efficacy - Mortality | Severe Cases |
| 2020-00210 6-68 |
Favipiravir, lopinavir/ritonavir or combination therapy: a randomised, double blind, 2x2 factorial placebo-controlled trial of early antiviral therapy in COVID-19 | United Kingdom | Y | Y | Drug Repurposing | Favipiravir, Lopinavir/ritonavir | Efficacy - Viral load | Mild/Moderate Cases |
| 2020-00185 4-23 |
Cumulative adaptive, multiarm, multistage and multicentre randomized clinical trial with immunotherapy for Moderate COVID-19 | Italy | Y | Y | Drug Repurposing | Siltuximab, Tocilizumab, Canakinumab, Baricitinib, Methylprednisolone, Sarilumab | Efficacy - Oxygen parameters | Mild/Moderate Cases |
| 2020-00140 8-41 |
A prospective, randomized, double blinded placebo-controlled trial to evaluate the efficacy and safety of tocilizumab in patients with severe COVID-19 pneumonia | Germany | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Other efficacy | Severe Cases |
| 2020-00248 2-34 |
Efficacy and safety of Octagam 10% therapy in COVID-19 patients with severe disease progression | United States | Y | N | Cell/Blood based | ND:Octagam | Efficacy - WHO scale | Severe Cases |
| 2020-00130 6-35 |
Protective role of inhaled steroids for COVID-19 infection | France | Y | N | Drug Repurposing | Budesonide, Formoterol | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| 2020-00102 3-14 |
A randomised double-blind placebo-controlled trial to determine the safety and efficacy of inhaled SNG001 (IFNß-1a for nebulisation) for the treatment of patients with confirmed SARS-CoV-2 infection (COVID-19) | United Kingdom | Y | N | Drug Repurposing | Interferon beta-1a | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| 2020-00154 1-39 |
Pilot study of single-dose bevacizumab as a treatment for acute respiratory distress syndrome in patients with COVID-19 | Spain | N | N | Drug Repurposing | Bevacizumab | Efficacy - Mortality | Severe Cases |
| 2020-00136 3-85 |
COVID-19 Prophylaxis with hydroxychloroquine, Vitamin D, and Zinc supplementation in Danish nursing home residents – a randomized controlled trial | Denmark | Y | N | Drug Repurposing | Hydroxychloroquine, Zinc, Vitamin D3 | Efficacy - Infection rate (prevention) | General population |
| 2020-00163 6-95 |
Subcutaneous and Intravenous anakinra in COVID-19 Infection - Feasibility & Pharmacokinetics/ Pharmacodynamics study | United Kingdom | Y | N | Drug Repurposing | Anakinra | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| 2020-00176 7-86 |
An open-label, multi-centre, randomised trial comparing different doses of single-dose tocilizumab in adults with severe, non-critical, PCR-confirmed COVID-19 infection with evidence of progressive decline in respiratory function and evolving systemic inflammation on time to intubation, non-invasive ventilation and/or all-cause mortality | Ireland | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| 2020-00139 1-15 |
A randomized double-blind placebo-controlled trial of intravenous plasma-purified alpha-1 antitrypsin for severe COVID-19 illness. | Ireland | N | N | Cell/Blood based | ND:Alpha-1 antitrypsin | Efficacy - Other efficacy | Severe Cases |
| 2020-00136 4-29 |
Phase I/II clinical trial to evaluate the safety and efficacy of Allogenic Adipose Tissue-Derived Mesenchymal Stem Cells Expanded in patients with severe COVID-19 pneumonia | Spain | Y | N | Cell/Blood based | ND:Stem cells | Safety | Severe Cases |
| 2020-00178 6-36 |
Recombinant InterLeukin-7 (CYT107) to Improve clinical outcomes in lymphopenic pAtients with COVID-19 infection “ILIAD 7 trial” | France | Y | N | Drug Repurposing | CYT107 | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| 2020-00231 2-43 |
Clinical trial, PHASE III, randomized, open-label, to evaluate the efficacy of administering high-dose cholecalciferol orally alongside standard therapy in patients with COVID-19 pneumonia (COVID-19 HUSO). | Spain | Y | N | Drug Repurposing | Vitamin D3 | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| 2020-00197 2-13 |
Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalised patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD) | Italy | Y | N | Drug Repurposing | Enoxaparin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| 2020-00149 8-63 |
Adaptive design phase 2 to 3, randomized, double- blind, multicenter, to evaluate the safety, efficacy, pharmacokinetics and pharmacodynamics of BIO101 in the prevention of the respiratory deterioration in hospitalized patients with COVID-19 pneumonia (severe stage) | France | Y | N | Shelved drug or NME | BIO101 | Efficacy - Other efficacy | Severe Cases |
| 2020-00186 0-27 |
AGILE: Seamless Phase I/IIa Platform for the Rapid Evaluation of Candidates for COVID-19 treatment | United Kingdom | Y | N | Shelved drug or NME | Nomacopan | Safety | Mild/Moderate Cases, Severe Cases |
| 2020-00128 1-11 |
Evaluation of the concentration/viral effect relationship of hydroxychloroquine in COVID-19 patients in the intensive care unit. | France | N | N | Drug Repurposing | Hydroxychloroquine | Other | Severe Cases |
| 2020-00189 1-14 |
Impact of the use of low molecular weight heparins (LMWH), at prophylactic versus intermediate doses, on SARS-CoV2 infection (COVID-19) | Spain | Y | N | Drug Repurposing | Enoxaparin | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| 2020-00159 8-66 |
Preliminary randomized controlled trial of poractant alfa (Curosurf®) by fiberoptic bronchoscopy-directed endobronchial administration in acute respiratory distress syndrome (ARDS) due to COVID-19 viral pneumonia. | France | Y | N | Drug Repurposing | Poractant alfa | Efficacy - Oxygen parameters | Severe Cases |
| 2020-00143 1-27 |
Treatment of Sars-CoV2 infections (COVID-19) with valsartan vs placebo, a three-armed randomized, partly blinded trial | Germany | Y | N | Drug Repurposing | Valsartan | Efficacy - WHO scale | Mild/Moderate Cases, Other |
| 2020-00144 2-19 |
Pilot, randomized, multicenter, open-label clinical trial of combined use of hydroxychloroquine, azithromycin, and tocilizumab for the treatment of SARS-CoV-2 infection (COVID-19) | Spain | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Mortality | Mild/Moderate Cases |
| 2020-00162 2-64 |
OUTPATIENT TREATMENT OF COVID-19 WITH EARLY PULMONARY CORTICOSTEROIDS AS AN OPPORTUNITY TO MODIFY THE COURSE OF THE DISEASE | Spain | Y | N | Drug Repurposing | Prednisone | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| 2020-00182 7-15 |
EARLY TREATMENT OF PNEUMONIA COVID-19 WITH GLUCOCORTICOIDS. RANDOMIZED CONTROLLED CLINICAL TRIAL | Spain | Y | N | Drug Repurposing | Methylprednisolone | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| 2020-00103 1-27 |
Treatment of non-severe confirmed cases of COVID-19 and chemoprophylaxis of their contacts as prevention strategy: a Cluster Randomized Clinical Trial (PEP CoV-2 Study) | Spain | Y | N | Drug Repurposing | Darunavir | Efficacy - Infection rate (prevention) | Mild/Moderate Cases |
| 2020-00175 4-21 |
An open prospective randomized therapeutic trial using ANAKINRA or TOCILIZUMAB alone or in combination with RUXOLITINIB in severe stage 2b and 3 COVID-19 disease | France | Y | N | Drug Repurposing | Anakinra, Tocilizumab, Ruxolitinib | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| 2020-00111 3-21 |
Randomised Evaluation of COVID-19 Therapy (RECOVERY) | United Kingdom | Y | Y | Drug Repurposing | Lopinavir/ritonavir, Dexamethasone, Azithromycin, Tocilizumab | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| 2020-00145 0-22 |
Phase II Clinical Trial to explore the efficacy of allogeneic mesenchymal cells from umbilical cord tissue in patients with severe pulmonary involvement by COVID-19. | Spain | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Mortality | Severe Cases |
| 2020-00089 0-25 |
Treatment of Coronavirus SARS-Cov2 Respiratory Infections with Hydroxychloroquine | France | N | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| 2020-00170 9-21 |
Effectiveness of low molecular weight heparin at increased doses prophylaxis weight-adjusted, compared with lower doses prophylaxis (intermediate or standard), on the onset of venous thromboembolism in coronavirus disease 2019 (COVID-19) hospitalized patients : The randomized multicentric controlled open-label trial COVI-DOSE | France | Y | N | Drug Repurposing | Enoxaparin, Tinzaparin, Nadroparin | Other | Mild/Moderate Cases |
| 2020-00125 5-40 |
Sarilumab Treatment In cytoKinE storm caused by infection with COVID-19. | Spain | N | N | Drug Repurposing | Sarilumab | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| 2020-00172 2-66 |
Plasma turnover in patients with COVID-19 disease and invasive mechanical ventilation: a randomized study | Spain | Y | N | Drug Repurposing | Albumin | Efficacy - Mortality | Severe Cases |
| 2020-00161 4-38 |
Covid-19: A randomized, open-label, adaptive, proof-of- concept clinical trial of new antiviral drug candidates against SARS-CoV-2. | Belgium | Y | N | Drug Repurposing | Azithromycin | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| 2020-00132 1-31 |
Prospective, phase II, randomized, open-label, parallel group study to evaluate the efficacy of hydroxychloroquine together with baricitinib, imatinib or early lopinavir / ritonavir in patients with SARS Cov2 pneumonia (COVID-19 HUF) | Spain | Y | Y | Drug Repurposing | Hydroxychloroquine, Baricitinib, Imatinib, Lopinavir/ritonavir | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| 2020-00153 1-27 |
Clinical trial of Sarilumab in adults hospitalized with COVID-19 presenting cytokine release syndrome | Spain | Y | N | Drug Repurposing | Sarilumab | Efficacy - Oxygen parameters | Severe Cases |
| 2020-00142 0-34 |
Senicapoc in COVID-19 Patients with Severe Respiratory Insufficiency – A Randomized, Open-Label, Phase II Trial | Denmark | Y | N | Shelved drug or NME | Senicapoc | Efficacy - Oxygen parameters | Severe Cases |
| 2020-00124 6-18 |
Cohort Multiple randomized controlled trials open-label of immune modulatory drugs and other treatments in COVID-19 patients | France | Y | N | Drug Repurposing | Sarilumab, Tocilizumab, Nivolumab, Bevacizumab, Tinzaparin, Hydroxychloroquine, Eculizumab, Baricitinib, Secukinumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| 2020-00124 4-26 |
COVID-19: Efficacy of solnatide to treat pulmonary permeability oedema in SARS-Cov-2 positive patients with moderate-to-severe ARDS - a pilot-trial. | Austria | Y | N | Shelved drug or NME | Solnatide | Efficacy - Other efficacy | Severe Cases |
| 2020-00139 0-76 |
A phase 3, randomized, open-labeled, multi-center study comparing clinical efficacy and safety of intravenous sarilumab plus standard of care compared to standard of care, in the treatment of patients with severe COVID-19 pneumonia | Italy | Y | N | Drug Repurposing | Sarilumab | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| 2020-00131 0-38 |
A randomized, prospective, open label clinical trial on the use of convalescent plasma compared to best supportive care in patients with severe COVID-19 | Germany | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| 2020-00271 3-17 |
A PHASE II, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, MULTICENTER STUDY TO EVALUATE THE SAFETY AND EFFICACY OF MSTT1041A OR UTTR1147A IN PATIENTS WITH SEVERE COVID-19 PNEUMONIA | Spain | Y | Y | Shelved drug or NME | MSTT1041A, UTTR1147A | Efficacy - WHO scale | Severe Cases |
| 2020-00140 9-21 |
A prospective, multicenter, randomized, parallel, double-blind, placebo-controlled phase IIb clinical trial to evaluate intravenous infusion of Defibrotide in the prevention and treatment of respiratory distress and cytokine release syndrome in patients with COVID-19. | Spain | Y | N | Drug Repurposing | Defibrotide | Efficacy - Mortality | Severe Cases |
| 2020-00173 2-10 |
Ruxolitinib for treatment of Covid-19 induced lung injury ARDS (RuXoCoil) A single-arm, open-label, proof of concept study | Germany | N | N | Drug Repurposing | Ruxolitinib | Efficacy - Mortality | Severe Cases |
| 2020-00163 2-10 |
A Randomized Open label Phase-II Clinical Trial with or without Infusion of Plasma from Subjects after Convalescence of SARS-CoV-2 Infection in High-Risk Patients with Confirmed Severe SARS-CoV-2 Disease | Germany | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - WHO scale | Severe Cases |
| 2020-00219 3-27 |
Double-blind, randomized, controlled, clinical trial to assess the efficacy of allogenic mesenchymal stromal cells in patients with acute respiratory distress syndrome due to COVID-19 | Spain | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Oxygen parameters | Severe Cases |
| 2020-00115 6-18 |
Randomized clinical trial to evaluate the efficacy of different treatments in patients with COVID-19 who require hospitalization | Spain | Y | Y | Drug Repurposing | Lopinavir/ritonavir, Hydroxychloroquine, Azithromycin | Efficacy - Mortality | Severe Cases |
| 2019-00268 8-89 |
Phase 1/2 clinical study to assess the feasibility, safety, tolerability and preliminary efficacy of the administration of HCR040, a drug whose active substance is HC016, allogeneic adipose-derived adult mesenchymal stem cells expanded and pulsed with H2O2, in patients with acute respiratory distress syndrome. (included patients COVID-19) | Spain | Y | N | Cell/Blood based | ND:Stem cells | Safety | Severe Cases |
| 2020-00176 8-27 |
"STUDY OF THE EFFICIENCY OF NORMAL HUMAN IMMUNOGLOBULINS (IVIG) IN PATIENTS AGED 75 YEARS AND OVER COVID-19 WITH SEVERE ACUTE RESPIRATORY FAILURE" GERONIMO 19 | France | N | N | Cell/Blood based | Immunoglobulin | Efficacy - Mortality | Severe Cases |
| 2020-00131 9-26 |
Multicenter, randomized, controlled, open-label clinical trial to assess the prognostic implications of rosuvastatin treatment in patients discharged after hospitalization for COVID-19 Positive. | Spain | Y | N | Drug Repurposing | Rosuvastatin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| 2020-00137 9-34 |
Sedation with sevoflurane versus propofol in patients with Acute Respiratory Distress Syndrome caused by COVID-19 infection | Spain | Y | N | Drug Repurposing | Sevoflurane | Efficacy - Oxygen parameters | Severe Cases |
| 2020-00125 7-51 |
The Danish Pre-HCQ Dialysis Study: Hydroxychloroquine for prevention of COVID-19 in dialysis-treated patients with end-stage renal disease - A multicenter parallel-group open randomized clinical trial | Denmark | Y | N | Drug Repurposing | Hydroxychloroquine | Other | Other |
| 2020-00197 1-33 |
Pragmatic study "CORIVER": Ivermectin as antiviral treatment for patients infected by SARS-COV2 (COVID-19) | Spain | Y | N | Drug Repurposing | Ivermectin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| 2020-00126 6-11 |
Two-center, randomized, controlled clinical trial with two treatment arms to evaluate the safety and efficacy of intravenous administration of expanded allogeneic adipose tissue adult mesenchymal cells in critically ill patients COVID-19. | Spain | Y | N | Cell/Blood based | ND:MSC | Efficacy - Mortality | Severe Cases |
| 2020-00171 7-20 |
Prevention and treatment with Calcifediol of Coronavirus COVID-19-induced acute respiratory syndrome (SARS) | Spain | Y | N | Drug Repurposing | Calcifediol | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| 2020-00157 3-78 |
Recombinant InterLeukin-7 (CYT107) to Improve clinical outcomes in lymphopenic pAtients with COVID-19 infection | United States | Y | N | Shelved drug or NME | CYT-107 | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| 2020-00130 3-16 |
Efficacy of Hydroxychloroquine, Telmisartan and Azithromycin on Survival in Elderly Hospitalized Patients with VIDOC-19 : A Randomized, Multi-Centre, Adaptive, Blinded Study | France | Y | Y | Drug Repurposing | Hydroxychloroquine, Telmisartan, Azithromycin | Efficacy - Mortality | Severe Cases, Other |
| 2020-00151 1-25 |
Randomized open-blind controlled trial to study the benefit of Colchicine in Patients with COVID-19 | Spain | Y | N | Drug Repurposing | Colchicine | Efficacy - WHO scale | Severe Cases |
| 2020-00140 6-27 |
Randomized open label trial assessing efficacy and safety of hydroxychloroquine plus azithromycin versus hydroxychloroquine for hospitalized patients with COVID-19 | France | Y | N | Drug Repurposing | Azithromycin | Efficacy - WHO scale | Severe Cases |
| 2020-00190 3-17 |
A randomized clinical trial (IIIb) of eficacy of a single dose of Tocilizumab or a combination of Tocilizumab plus Vitamin D (single i.m. dose) for the treatment of the COVID-19 hyperimmune complication. Assessment of IL-6. | Spain | Y | Y | Drug Repurposing | Tocilizumab, Vitamin D3 | Efficacy - Mortality | Severe Cases |
| 2020-00126 5-36 |
A multicentre, prospective, randomised trial comparing standard of care (SOC) alone, SOC plus hydroxychloroquine monotherapy or SOC plus a combination of hydroxychloroquine and azithromycin in the treatment of non-critical, SARS-CoV-2 PCR-positive population not requiring immediate resuscitation or ventilation but who have evidence of clinical decline. | Ireland | Y | Y | Drug Repurposing | Azithromycin, Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| 2020-00103 8-36 |
A Multi-site Phase I/II, 2-Part, Dose-Escalation Trial Investigating the Safety and Immunogenicity of four Prophylactic SARS-CoV-2 RNA Vaccines Against COVID-19 Using Different Dosing Regimens in Healthy Adults | Germany | N | N | Vaccine | BNT162 vaccine | Safety | General population |
| 2020-00203 7-15 |
Multicenter, randomized, open-label study to evaluate the efficacy and safety of SOC + Sarilumab versus Standard of Care for the Early Treatment of COVID-19-pneumonia in Hospitalized Patients | Spain | Y | N | Drug Repurposing | Sarilumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| 2020-00295 2-18 |
Targeting de novo Pyrimidine Biosynthesis by leflunomide as a Novel Concept for the Treatment of Corona Virus Disease 2019 (COVID-19) | United Kingdom | Y | N | Drug Repurposing | Leflunomide | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| 2020-00173 9-28 |
A randomized, open-label, adaptive, proof-of-concept clinical trial of modulation of host thromboinflammatory response in patients with COVID-19. | Belgium | Y | N | Drug Repurposing | Aprotinin, Anakinra | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| 2020-00130 7-16 |
Efficacy and Safety of corticoids in patients with adult respiratory distress syndrome (ARDS) secondary to COVID-19. | Spain | Y | N | Drug Repurposing | Methylprednisolone | Efficacy - Mortality | Severe Cases |
| 2020-00173 6-95 |
ACCORD 2: A Multicentre, Seamless, Phase 2 Adaptive Randomisation Platform Study to Assess the Efficacy and Safety of Multiple Candidate Agents for the Treatment of COVID 19 in Hospitalised Patients | United Kingdom | Y | N | Drug Repurposing, Shelved drug or NME | Bemcentinib, MEDI3506, Acalabrutinib, Zilucoplan | Efficacy - WHO scale | Severe Cases |
| 2020-00126 2-11 |
Randomized, controlled, blinded clinical trial for the evaluator, to evaluate the efficacy and safety of treatment with cyclosporine A (CsA) associated with standard treatment versus standard treatment only in hospitalized patients with confirmed infection by COVID-19 | Spain | Y | N | Drug Repurposing | Cyclosporine | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| 2020-00150 5-22 |
Double-blind, randomized, parallel, placebo-controlled pilot clinical trial, nested in a prospective cohort observational study, for the evaluation of the efficacy and safetyof two doses of WJ-MSC in patients with acute respiratory distress syndrome secondary to infection by COVID-19 | Spain | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| 2020-00129 0-74 |
Efficacy and safety of sarilumab in the early treament of hospitalized patients with mild-moderate neumonia and COVID19 infection versus standard of care | Spain | Y | N | Drug Repurposing | Sarilumab | Efficacy - WHO scale | Mild/Moderate Cases |
| 2020-00141 3-20 |
Phase 2, randomized, open-label study to compare the efficacy and safety of siltuximab vs. corticosteroids in hospitalized patients with COVID19 pneumonia | Spain | Y | N | Drug Repurposing | Siltuximab, Methylprednisolone | Efficacy - Other efficacy | Severe Cases |
| 2020-00170 7-16 |
PHASE III RANDOMIZED, UNICENTRIC, OPEN, CONTROLLED CLINICAL TRIAL TO DEMONSTRATE THE EFFECTIVENESS OF TOCILIZUMAB AGAINST SYSTEMIC CORTICOTHERAPY IN PATIENTS ENTERED BY COVID-19 WITH BILATERAL PNEUMONIA AND BAD EVOLUTION | Spain | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| 2020-00263 2-75 |
Multicenter, open-label, randomised trial to assess the efficacy and tolerability of poractant alfa (porcine surfactant, Curosurf®) in hospitalized patients with SARS-COV-19 acute respiratory distress syndrome (ARDS) | United States | Y | N | Drug Repurposing | Poractant alfa | Efficacy - Oxygen parameters | Severe Cases |
| 2020-00140 5-23 |
Randomized phase II clinical trial of ruxolitinib plus simvastatin in the prevention and treatment of respiratory failure of COVID-19.Ruxo-Sim-20 clinical trial. | Spain | Y | N | Drug Repurposing | Ruxolitinib, Simvastatin | Efficacy - WHO scale | Severe Cases |
| NCT04372602 | Duvelisib to Combat COVID-19 | United States | N | N | Drug Repurposing | Duvelisib | Efficacy - Mortality | Severe Cases |
| NCT04482621 | Decitabine for Coronavirus (COVID-19) Pneumonia- Acute Respiratory Distress Syndrome (ARDS) Treatment: DART Trial | United States | Y | N | Drug Repurposing | Decitabine | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04425538 | A Phase 2 Trial of Infliximab in Coronavirus Disease 2019 (COVID-19). | United States | N | N | Drug Repurposing | Infliximab | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04454307 | Safety and Efficacy of Tramadol in COVID-19 Egyptian Patients | Egypt | Y | N | Drug Repurposing | Tramadol | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04333550 | Application of Desferal to Treat COVID-19 | Iran | Y | N | Drug Repurposing | Deferoxamine | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04432987 | Dornase Alpha for the Treatment of COVID-19 | Turkey | Y | N | Drug Repurposing | Dornase alfa | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04336904 | Clinical Study To Evaluate The Performance And Safety Of Favipiravir in COVID-19 | Italy | Y | N | Drug Repurposing | Favipiravir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04359095 | Effectiveness and Safety of Medical Treatment for SARS-CoV-2 (COVID-19) in Colombia | Colombia | Y | Y | Drug Repurposing | Hydroxychloroquine, Lopinavir/ritonavir, Azithromycin | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04354831 | A Study Evaluating the Efficacy and Safety of High-Titer Anti-SARS-CoV-2 Plasma in Hospitalized Patients With COVID-19 Infection | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04445285 | Phase 2 Trial Using rhDNase to Reduce Mortality in COVID-19 Patients With Respiratory Failure | United States | Y | N | Drug Repurposing | Dornase alfa | Efficacy - Mortality | Severe Cases |
| NCT04345445 | Study to Evaluate the Efficacy and Safety of Tocilizumab Versus Corticosteroids in Hospitalised COVID-19 Patients With High Risk of Progression | Malaysia | Y | N | Drug Repurposing | Tocilizumab, Methylprednisolone | Efficacy - Oxygen parameters | Severe Cases |
| NCT04476888 | Convalescent Plasma Treatment in COVID-19 | Pakistan | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04366739 | Repurposing of Chlorpromazine in Covid-19 Treatment | France | Y | N | Drug Repurposing | Chlorpromazine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04366960 | Comparison of Two Doses of Enoxaparin for Thromboprophylaxis in Hospitalized COVID-19 Patients | Italy | Y | Y | Drug Repurposing | Enoxaparin | Efficacy - Infection rate (prevention) | Mild/Moderate Cases, Severe Cases |
| NCT04361422 | Isotretinoin in Treatment of COVID-19 | Egypt | Y | N | Drug Repurposing | Isotretinoin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04405310 | Convalescent Plasma of Covid-19 to Treat SARS-COV-2 a Randomized Doble Blind 2 Center Trial | Mexico | Y | Y | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| NCT04328272 | Effectiveness of Hydroxychloroquine in Covid-19 Patients | Pakistan | Y | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04345679 | Anti COVID-19 Convalescent Plasma Therapy | Hungary | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Viral load | Severe Cases |
| NCT04461340 | Efficacy and Safety of Sirolimus in COVID-19 Infection | Egypt | Y | N | Drug Repurposing | Sirolimus | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04428801 | Autologous Adipose-derived Stem Cells (AdMSCs) for COVID-19 | United States | Y | N | Cell/Blood based | ND:Stem cells | Safety | Other |
| NCT04321421 | Hyperimmune Plasma for Critical Patients With COVID-19 | Italy | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| NCT04370288 | Clinical Application of Methylene Blue for Treatment of Covid-19 Patients | Iran | Y | N | Drug Repurposing | Methylene blue, Ascorbic acid, N-acetylcysteine | Efficacy - Oxygen parameters | Severe Cases |
| NCT04347915 | The Phase 2 Study to Evaluate the Safety and Efficacy of Clevudine in Patients With Moderate COVID-19 | Korea, Republic of | Y | N | Drug Repurposing | Clevudine | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04333407 | Preventing Cardiac Complication of COVID-19 Disease With Early Acute Coronary Syndrome Therapy: A Randomised Controlled Trial. | United Kingdom | Y | Y | Drug Repurposing | Aspirin, Clopidogrel, Rivaroxaban, Atorvastatin, Omeprazole | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04473690 | KBP-201 COVID-19 Vaccine Trial in Healthy Volunteers | United States | Y | N | Vaccine | KBP-201 Vaccine | Safety | General population |
| NCT04470622 | Aprepitant Injectable Emulsion in Patients With COVID-19 (GUARDS-1) | United States | Y | N | Drug Repurposing | Aprepitant | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04401423 | TXA COVID-19 Clinical Trial | United States | Y | N | Shelved drug or NME | TXA127 | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04412486 | COVID-19 Convalescent Plasma (CCP) Transfusion | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Oxygen parameters | Severe Cases |
| NCT04452669 | VentaProst in Subjects With COVID-19 Requiring Mechanical Ventilation | United States | N | N | Drug Repurposing | Epoprostenol | Efficacy - Oxygen parameters | Severe Cases |
| NCT04406389 | Anticoagulation in Critically Ill Patients With COVID-19 (The IMPACT Trial) | United States | Y | Y | Drug Repurposing | Enoxaparin, Heparin, Fondaparinux, Argatroban | Efficacy - Mortality | Severe Cases |
| NCT04463472 | Study of COVID-19 DNA Vaccine (AG0301-COVID19) | Japan | N | N | Vaccine | AG0301 Vaccine | Safety | General population |
| NCT04474483 | Safety and Efficacy of Melatonin in Outpatients Infected With COVID-19 | United States | Y | N | Drug Repurposing | Melatonin | Safety | Mild/Moderate Cases |
| NCT04393038 | ABX464 in Treating Inflammation and Preventing Acute Respiratory Failure in Patients With COVID-19 | France | Y | N | Shelved drug or NME | ABX464 | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04434248 | An Adaptive Study of Favipiravir Compared to Standard of Care in Hospitalized Patients With COVID-19 | Russian Federation | Y | N | Drug Repurposing | Favipiravir | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04466657 | Antioxidant Therapy for COVID-19 Study | Nigeria | Y | N | Drug Repurposing, Other | ND:Antioxidant supplements, N-acetylcysteine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04363346 | Study of FT516 for the Treatment of COVID-19 in Hospitalized Patients With Hypoxia | United States | N | N | Shelved drug or NME | FT516 | Efficacy - Other efficacy | Severe Cases |
| NCT04372979 | Efficacy of Convalescent Plasma Therapy in the Early Care of COVID-19 Patients. | France | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04456413 | Convalescent Plasma as Treatment for Subjects With Early COVID-19 Infection | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04333420 | Open-label, Randomized Study of IFX-1 in Patients With Severe COVID-19 Pneumonia | Netherlands | Y | N | Shelved drug or NME | IFX-1 | Efficacy - Oxygen parameters | Severe Cases |
| NCT04467151 | Administration of Anti-SARS-CoV-2 Convalescent Plasma in Hospitalized, Non-ICU Patients With COVID-19 | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04479358 | Low-dose Tocilizumab Versus Standard of Care in Hospitalized Patients With COVID-19 | United States | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04343755 | Convalescent Plasma as Treatment for Hospitalized Subjects With COVID-19 Infection | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04464395 | Study of CPI-006 as Immunotherapy for Hospitalized COVID-19 Patients | United States | N | N | Shelved drug or NME | CPI-006 | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04324489 | DAS181 for Severe COVID-19: Compassionate Use | China | N | N | Shelved drug or NME | DAS181 | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04432298 | Study of the Efficacy and Safety of Intravenous Pamrevlumab, in Hospitalized Patients With Acute COVID-19 Disease | United States | Y | N | Shelved drug or NME | Pamrevlumab | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04449965 | Povidone-Iodine Rinses in the Management of COVID-19 | Canada | Y | N | Shelved drug or NME | Povidone-iodine | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04392141 | Colchicine Plus Phenolic Monoterpenes to Treat COVID-19 | Iran | Y | N | Drug Repurposing, Other | Colchicine, ND:Dietary supplement | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04370262 | Multi-site Adaptive Trials for COVID-19 | United States | Y | N | Drug Repurposing | Famotidine | Efficacy - Mortality | Severe Cases |
| NCT04382586 | Covid-19 Infection and Pulmonary Distress Treatment With Zanubrutinib in Hospitalized Participants | United States | Y | N | Drug Repurposing | Zanubrutinib | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04459286 | The Nitazoxanide Plus Atazanavir for COVID-19 Study | Nigeria | Y | N | Drug Repurposing | Nitazoxanide, Atazanavir/ritonavir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04329611 | ALBERTA HOPE COVID-19 for the Prevention of Severe COVID19 Disease | Canada | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04335123 | Study of Open Label Losartan in COVID-19 | United States | N | N | Drug Repurposing | Losartan | Safety | Severe Cases |
| NCT04321993 | Treatment of Moderate to Severe Coronavirus Disease (COVID-19) in Hospitalized Patients | Canada | Y | Y | Drug Repurposing | Baricitinib | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04376788 | Exchange Transfusion Versus Plasma From Convalescent Patients With Methylene Blue in Patients With COVID-19 | Egypt | Y | Y | Drug Repurposing, Cell/Blood based | Methylene blue, ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04360356 | Ivermectin and Nitazoxanide Combination Therapy for COVID-19 | Egypt | Y | N | Drug Repurposing | Ivermectin, Nitazoxanide | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04350593 | Dapagliflozin in Respiratory Failure in Patients With COVID-19 | United States | Y | N | Drug Repurposing | Dapagliflozin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04361318 | Hydroxychloroquine and Nitazoxanide Combination Therapy for COVID-19 | Egypt | Y | N | Drug Repurposing | Hydroxychloroquine, Nitazoxanide | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04375046 | Recombinant Bacterial ACE2 Receptors -Like Enzyme of B38-CAP Could be Promising COVID-19 Infection- and Lung Injury Preventing Drug Better Than Recombinant Human ACE2 | Egypt | Y | N | Shelved drug or NME | rbACE2 | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04420299 | Clinical Trial on the Efficacy and Safety of Bemiparin in Patients Hospitalized Because of COVID-19 | Spain | Y | N | Drug Repurposing | Bemiparin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04425629 | Safety, Tolerability, and Efficacy of Anti-Spike (S) SARS-CoV-2 Monoclonal Antibodies for the Treatment of Ambulatory Adult Patients With COVID-19 | United States | Y | N | Shelved drug or NME | REGN10987, REGN10933 | Safety | Mild/Moderate Cases |
| NCT04352933 | PROLIFIC ChemoprophylaxisTrial (COVID-19) | United Kingdom | Y | Y | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04364022 | Efficacy of Pragmatic Same-day COVID-19 Ring Prophylaxis for Adult Individuals Exposed to SARS-CoV-2 in Switzerland | Switzerland | Y | Y | Drug Repurposing | Hydroxychloroquine, Lopinavir/ritonavir | Efficacy - Other efficacy | Exposed individuals (family members, HCP) |
| NCT04347889 | Preventing COVID-19 in Healthcare Workers With HCQ: A RCT | United States | Y | N | Drug Repurposing | Hydroxychloroquine, Ascorbic acid | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04405102 | COVID-19 Ozanimod Intervention Study | Canada | Y | N | Drug Repurposing | Ozanimod | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04358081 | Hydroxychloroquine Monotherapy and in Combination With Azithromycin in Patients With Moderate and Severe COVID-19 Disease | United States | Y | Y | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04381052 | Study for the Use of the IL-6 Inhibitor Clazakizumab in Patients With Life-threatening COVID-19 Infection | United States | N | N | Shelved drug or NME | Clazakizumab | Safety | Severe Cases |
| NCT04325633 | Efficacy of Addition of Naproxen in the Treatment of Critically Ill Patients Hospitalized for COVID-19 Infection | France | Y | N | Drug Repurposing | Naproxen | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04486001 | Study of Intravenous Administration of Allogeneic Adipose Stem Cells for COVID-19 | United States | N | N | Cell/Blood based | ND:Stem cells | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04464408 | Favipiravir Therapy in Adults With Mild COVID-19 | Saudi Arabia | Y | N | Drug Repurposing | Favipiravir | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04382066 | Proof of Concept Study to Evaluate the Safety Profile of Plitidepsin in Patients With COVID-19 | Spain | Y | Y | Shelved drug or NME | Plitidepsin | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04408456 | Efficacy of Hydroxychloroquine (HCQ) as Post Exposure Prophylaxis (PEP) for Prevention of COVID-19 | India | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04380532 | Tableted COVID-19 Therapeutic Vaccine | Canada | N | N | Shelved drug or NME | ND:Convalescent plasma | Safety | General population |
| NCT04432103 | Treatment of Severe and Critical COVID-19 Pneumonia With Convalescent Plasma | Mexico | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Severe Cases |
| NCT04390178 | Convalescent Plasma as Treatment for Acute Coronavirus Disease (COVID-19) | Sweden | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04415086 | Treatment of Patients With COVID-19 With Convalescent Plasma | Brazil | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04485130 | DISulfiram for COvid-19 (DISCO) Trial | United States | Y | N | Drug Repurposing | Disulfiram | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04385043 | Hyperimmune Plasma in Patients With COVID-19 Severe Infection | Italy | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| NCT04317092 | Tocilizumab in COVID-19 Pneumonia (TOCIVID-19) | Italy | N | N | Drug Repurposing | Tocilizumab | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04375202 | Colchicine in COVID-19: a Pilot Study | Italy | Y | N | Drug Repurposing | Colchicine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04339660 | Clinical Research of Human Mesenchymal Stem Cells in the Treatment of COVID-19 Pneumonia | China | Y | N | Shelved drug or NME | ND:Stem cells | Other | Severe Cases |
| NCT04465695 | Dual Therapy With Interferon Beta-1b and Clofazimine for COVID-19 | Hong Kong | Y | Y | Drug Repurposing | Interferon beta-1b, Clofazimine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04348071 | Safety and Efficacy of Ruxolitinib for COVID-19 | United States | N | N | Drug Repurposing | Ruxolitinib | Safety | Severe Cases |
| NCT04340232 | Safety and Efficacy of Baricitinib for COVID-19 | United States | N | N | Drug Repurposing | Baricitinib | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04329832 | Hydroxychloroquine vs. Azithromycin for Hospitalized Patients With Suspected or Confirmed COVID-19 | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04388410 | Safety and Efficacy of Convalescent Plasma Transfusion for Patients With COVID-19 | Mexico | Y | N | Cell/Blood based | Convalescent plasma | Efficacy - Mortality | Severe Cases |
| NCT04489446 | Sildenafil in COVID-19 | Chile | Y | N | Drug Repurposing | Sildenafil | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04426695 | Safety, Tolerability, and Efficacy of Anti-Spike (S) SARS-CoV-2 Monoclonal Antibodies for Hospitalized Adult Patients With COVID-19 | United States | Y | N | Shelved drug or NME | REGN10987, REGN10933 | Safety | Severe Cases |
| NCT04467047 | Safety and Feasibility of Allogenic MSC in the Treatment of COVID-19 | Brazil | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04480632 | Therapeutic Plasmapheresis in Critically Ill Adult Patients With COVID-19 Confirmed Diagnosis | Colombia | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| NCT04443270 | Chloroquine Phosphate Prophylactic Use in Health Personnel Exposed to COVID-19 Patients | Mexico | N | N | Drug Repurposing | Chloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04324996 | A Phase I/II Study of Universal Off-the-shelf NKG2D-ACE2 CAR-NK Cells for Therapy of COVID-19 | China | N | Y | Cell/Blood based | ND:T-cells | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04384497 | Convalescent Plasma for Treatment of COVID-19: An Exploratory Dose Identifying Study | Sweden | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04354597 | Hydroxychloroquine and Azithromycin as Prophylaxis for Healthcare Workers Dealing With COVID19 Patients | Jordan | Y | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04410354 | Study of Merimepodib in Combination With Remdesivir in Adult Patients With Advanced COVID-19 | United States | Y | N | Shelved drug or NME | Merimepodib | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04371406 | Efficacy of Azithromycin-associated Hydroxychloroquine Therapy Given in General Practice in Early-stage Disease in COVID-19 Patients | France | Y | N | Drug Repurposing | Azithromycin, Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04380688 | Acalabrutinib Study With Best Supportive Care Versus Best Supportive Care in Subjects Hospitalized With COVID-19. | United States | Y | N | Drug Repurposing | Acalabrutinib | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04449588 | Efficacy and Safety Study of BDB-001 in Severe COVID-19 With ALI/ARDS | China | Y | N | Shelved drug or NME | BDB-001 | Efficacy - Oxygen parameters | Severe Cases |
| NCT04346199 | Acalabrutinib Study With Best Supportive Care Versus Best Supportive Care in Subjects Hospitalized With COVID-19. | United States | Y | N | Drug Repurposing | Acalabrutinib | Efficacy - Other efficacy | Severe Cases |
| NCT04359615 | Favipiravir in Hospitalized COVID-19 Patients | Iran | Y | N | Drug Repurposing | Favipiravir | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04333355 | Safety in Convalescent Plasma Transfusion to COVID-19 | Mexico | N | N | Cell/Blood based | ND:Convalescent plasma | Safety | Severe Cases |
| NCT04421404 | Effects of COVID-19 Convalescent Plasma (CCP) on Coronavirus-associated Complications in Hospitalized Patients | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04359316 | Azithromycin in Hospitalized COVID-19 Patients | Iran | Y | N | Drug Repurposing | Azithromycin | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04381884 | Ivermectin Effect on SARS-CoV-2 Replication in Patients With COVID-19 | United States | Y | N | Drug Repurposing | Ivermectin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04352400 | Efficacy of Nafamostat in Covid-19 Patients (RACONA Study) | Italy | Y | N | Drug Repurposing | Nafamostat | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04364815 | The University of the Philippines Hydroxychloroquine PEP Against COVID-19 Trial | Philippines | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04343768 | An Investigation Into Beneficial Effects of Interferon Beta 1a, Compared to Interferon Beta 1b And The Base Therapeutic Regiment in Moderate to Severe COVID-19: A Randomized Clinical Trial | Iran | Y | Y | Drug Repurposing | Hydroxychloroquine, Lopinavir/ritonavir, Interferon Beta-1A, Interferon Beta-1B | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04365231 | Hydroxychloroquine Azithromycin COVID-19 Pregnancy Trial | France | Y | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04428008 | Thymosin Alpha 1 to Prevent COVID-19 Infection in Elderly Renal Dialysis Patients | United States | Y | N | Drug Repurposing | Thymalfasin | Efficacy - Infection rate (prevention) | Other |
| NCT04355936 | Telmisartan for Treatment of COVID-19 Patients | Argentina | Y | N | Drug Repurposing | Telmisartan | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04252118 | Mesenchymal Stem Cell Treatment for Pneumonia Patients Infected With COVID-19 | China | Y | N | Shelved drug or NME | ND:Stem cells | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04466670 | Hemostasis in COVID-19: an Adaptive Clinical Trial | Brazil | Y | Y | Drug Repurposing | Heparin, Aspirin, Enoxaparin | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04492475 | Adaptive COVID-19 Treatment Trial 3 (ACTT-3) | United States | Y | N | Drug Repurposing, Shelved drug or NME | Interferon beta-1a | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04438980 | Glucocorticoids in COVID-19 (CORTIVID) | Spain | Y | N | Drug Repurposing | Methylprednisolone | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04280705 | Adaptive COVID-19 Treatment Trial (ACTT) | United States | Y | N | Shelved drug or NME | Remdesivir | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04401579 | Adaptive COVID-19 Treatment Trial 2 (ACTT-2) | United States | Y | N | Drug Repurposing | Baricitinib | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04348656 | CONvalescent Plasma for Hospitalized Adults With COVID-19 Respiratory Illness (CONCOR-1) | Canada | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| NCT04475107 | The Efficacy and Safety of Pyramax in Mild to Moderate COVID-19 Patients | Korea, Republic of | Y | N | Drug Repurposing | Pyronaridine/Artesunate | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04343976 | Pegylated Interferon Lambda Treatment for COVID-19 | United States | Y | N | Shelved drug or NME | Peg-Interferon lambda | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04357808 | Efficacy of Subcutaneous Sarilumab in Hospitalised Patients With Moderate-severe COVID-19 Infection (SARCOVID) | Spain | Y | N | Drug Repurposing | Sarilumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04441385 | Study to Evaluate the Efficacy and Safety of Maraviroc in SARS-CoV-2 Infection (COVID-19). | Spain | Y | N | Drug Repurposing | Maraviroc | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04397328 | COVID-19 PEP- High-risk Individuals in Long-term and Specialized Care - Canada | Canada | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04330586 | A Trial of Ciclesonide in Adults With Mild COVID-19 | Korea, Republic of | Y | Y | Drug Repurposing | Ciclesonide, Hydroxychloroquine | Efficacy - Viral load | General population |
| NCT04414098 | Ruxolitinib in the Treatment of Covid-19 | Argentina | N | N | Drug Repurposing | Ruxolitinib | Efficacy - Oxygen parameters | Severe Cases |
| NCT04345523 | Convalescent Plasma Therapy vs. SOC for the Treatment of COVID19 in Hospitalized Patients | Spain | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - WHO scale | Severe Cases |
| NCT04374279 | Trial to Promote Recovery From COVID-19 With Endocrine Therapy | United States | Y | N | Drug Repurposing | Bicalutamide | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04349592 | Hydroxychloroquine With or Without Azithromycin for Virologic Cure of COVID-19 | Qatar | Y | N | Drug Repurposing | Hydoxychloroquine, Azithromycin | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04350671 | Interferon Beta 1a in Hospitalized COVID-19 Patients | Iran | Y | N | Drug Repurposing | Interferon beta-1A | Efficacy - WHO scale | Severe Cases |
| NCT04425252 | Safety and Anti-coronavirus Response of Suppression of Host Nucleotide Synthesis in Patients With COVID-19 | United States | Y | N | Drug Repurposing | Brequinar | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04365257 | Prazosin to Prevent COVID-19 (PREVENT-COVID Trial) | United States | Y | N | Drug Repurposing | Prazosin | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04379479 | Clinical Effect of Dialyzable Leukocyte Extract in Suspected or Confirmed Cases of COVID-19 (FUTURE-T) | Mexico | Y | N | Shelved drug or NME | Dialyzable Leukocyte Extract | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04466540 | Randomized Placebo-controlled Trial of Hydroxychloroquine in Outpatient Cases With Coronavirus Disease 2019 (COVID-19) | Brazil | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04350684 | Umifenovir in Hospitalized COVID-19 Patients | Iran | Y | N | Drug Repurposing | Umifenovir | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04328285 | Chemoprophylaxis of SARS-CoV-2 Infection (COVID-19) in Exposed Healthcare Workers | France | Y | Y | Drug Repurposing | Hydroxychloroquine, Lopinavir/ritonavir | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04299724 | Safety and Immunity of Covid-19 aAPC Vaccine | China | N | N | Vaccine | ND:Covid-19 aAPC vaccine | Safety | General population, Mild/Moderate Cases |
| NCT04456153 | Atovaquone for Treatment of COVID-19 | United States | Y | N | Drug Repurposing | Atovaquone | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04416048 | Effect of Anticoagulation Therapy on Clinical Outcomes in COVID-19 | Germany | Y | N | Drug Repurposing | Rivaroxaban | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04315298 | Evaluation of the Efficacy and Safety of Sarilumab in Hospitalized Patients With COVID-19 | United States | Y | Y | Drug Repurposing | Sarilumab | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04389359 | PROphylaxis for paTiEnts at Risk of COVID-19 infecTion | United Kingdom | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04418518 | A Trial of CONvalescent Plasma for Hospitalized Adults With Acute COVID-19 Respiratory Illness | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04366232 | Efficacy of Intravenous Anakinra and Ruxolitinib During COVID-19 Inflammation (JAKINCOV) | France | Y | N | Drug Repurposing | Ruxolitinib, Anakinra | Efficacy - Other efficacy | Severe Cases |
| NCT04363372 | A Study for Efficacy and Safety of Live Biotherapeutic MRx4DP0004 to Treat COVID-19 | United Kingdom | Y | N | Shelved drug or NME | MRx-4DP0004 | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04355897 | CoVID-19 Plasma in Treatment of COVID-19 Patients | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04377620 | Assessment of Efficacy and Safety of Ruxolitinib in Participants With COVID-19-Associated ARDS Who Require Mechanical Ventilation (RUXCOVID-DEVENT) | United States | Y | N | Drug Repurposing | Ruxolitinib | Efficacy - Mortality | Severe Cases |
| NCT04475991 | Safety and Efficacy of Maraviroc and/or Favipiravir vs Currently Used Therapy in Severe COVID-19 Adults | Mexico | Y | Y | Drug Repurposing | Maraviroc, Favipiravir | Efficacy - Other efficacy | Severe Cases |
| NCT04435184 | Crizanlizumab for Treating COVID-19 Vasculopathy | United States | Y | N | Drug Repurposing | Crizanlizumab | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04377750 | The Use of Tocilizumab in the Management of Patients Who Have Severe COVID-19 With Suspected Pulmonary Hyperinflammation | Israel | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Mortality | Severe Cases |
| NCT04448119 | Control of COVID-19 Outbreaks in Long Term Care | Canada | Y | N | Drug Repurposing | Favipiravir | Efficacy - Infection rate (prevention) | Other |
| NCT04353180 | Assessment the Activity Value of Isotretinoin (13- Cis-Retinoic Acid ) in the Treatment of COVID-19 (Randomized) | Egypt | Y | Y | Drug Repurposing | Retinoic acid | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04371965 | Povidone Iodine Mouthwash, Gargle, and Nasal Spray to Reduce Naso- Pharyngeal Viral Load in Patients With COVID-19 | France | Y | N | Shelved drug or NME | Povidone-Iodine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04363866 | A Pilot Study to Assess Hydroxychloroquine in Patients With SARS-CoV-2 (COVID-19) | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04330638 | Treatment of COVID-19 Patients With Anti-interleukin Drugs | Belgium | Y | Y | Drug Repurposing | Anakinra, Siltuximab, Tocilizumab | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04394442 | Hydroxychloroquine in COVID-19 Patients | Saudi Arabia | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04485429 | Efficacy Assessment of Methylprednisolone and Heparin in Patients With COVID-19 Pneumonia | Brazil | Y | Y | Drug Repurposing | Methylprednisolone, Heparin | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04382625 | Hydroxychloroquine in SARS-CoV-2 (COVID-19) Pneumonia Trial | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Oxygen parameters | Mild/Moderate Cases |
| NCT04353206 | Convalescent Plasma in ICU Patients With COVID-19-induced Respiratory Failure | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Other | Severe Cases |
| NCT04488081 | I-SPY COVID-19 TRIAL: An Adaptive Platform Trial for Critically Ill Patients | United States | Y | Y | Drug Repurposing | Cenicriviroc, Icatibant, Razuprotafib, Apremilast | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04473053 | Rapid Experimental Medicine for COVID-19 | United Kingdom | Y | Y | Drug Repurposing, Shelved drug or NME | TD139, Nafamostat | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04400890 | Randomized Proof-of-Concept Trial to Evaluate the Safety and Explore the Effectiveness of Resveratrol for COVID-19 | United States | Y | N | Drug Repurposing | Resveratrol | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04425837 | Effectiveness and Safety of Convalescent Plasma in Patients With High-risk COVID-19 | Colombia | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04452474 | Study of the Efficacy and Safety of a Single Administration of Olokizumab vs. Placebo in Addition to Standard Treatment in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection (COVID-19). | United States | Y | N | Shelved drug or NME | Olokizumab | Efficacy - WHO scale | Severe Cases |
| NCT04361214 | Leflunomide in Mild COVID-19 Patients | United States | N | N | Drug Repurposing | Leflunomide | Safety | Mild/Moderate Cases |
| NCT04380519 | Study of the Efficacy and Safety of a Single Administration of Olokizumab and RPH-104 With Standard Therapy in Patients With Severe Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection (COVID-19) | Russian Federation | Y | N | Shelved drug or NME | Olokizumab, RPH-104 | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04483635 | Preventing COVID-19 With High-dose Vitamin D Supplements | Canada | Y | N | Drug Repurposing | Vitamin D | Efficacy - Infection rate (prevention) | General population |
| NCT04313322 | Treatment of COVID-19 Patients Using Wharton's Jelly-Mesenchymal Stem Cells | Jordan | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04369742 | Treating COVID-19 With Hydroxychloroquine (TEACH) | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Safety | Severe Cases |
| NCT04394117 | Controlled evaLuation of Angiotensin Receptor Blockers for COVID-19 respIraTorY Disease | Australia | Y | N | Drug Repurposing | Angiotensin Receptor Blockers | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04389411 | The COvid-19 Symptom MOntelukast Trial | Canada | Y | N | Drug Repurposing | Montelukast | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04398303 | ACT-20 in Patients With Severe COVID-19 Pneumonia | United States | Y | Y | Cell/Blood based | ND:Stem cells | Efficacy - Mortality | Severe Cases |
| NCT04435795 | Inhaled Ciclesonide for Outpatients With COVID19 | United States | Y | N | Drug Repurposing | Ciclesonide | Efficacy - Oxygen parameters | Mild/Moderate Cases |
| NCT04425707 | Ivermectin In Treatment of COVID 19 Patients | Egypt | Y | N | Drug Repurposing | Ivermectin | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04355767 | Convalescent Plasma in Outpatients With COVID-19 | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04326920 | Sargramostim in Patients With Acute Hypoxic Respiratory Failure Due to COVID-19 (SARPAC) | Belgium | Y | N | Drug Repurposing | Sargramostim | Efficacy - Oxygen parameters | Mild/Moderate Cases |
| NCT04446377 | A Study of LAM-002A for the Prevention of Progression of COVID-19 | United States | Y | N | Shelved drug or NME | Apilimod dimesylate | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04304313 | A Pilot Study of Sildenafil in COVID-19 | China | N | N | Drug Repurposing | Sildenafil | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04390503 | Convalescent Plasma for COVID-19 Close Contacts | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Exposed individuals (family members, HCP), Mild/Moderate Cases |
| NCT04382755 | Zilucoplan® in Improving Oxygenation and Short- and Long-term Outcome of COVID-19 Patients With Acute Hypoxic Respiratory Failure | Belgium | Y | N | Shelved drug or NME | Zilucoplan | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04365699 | Cardiovascular Effects of COVID-19 | United States | N | N | Shelved drug or NME | AT-001 | Efficacy - Other efficacy | Other |
| NCT04458298 | A Study to Evaluate OP-101 (Dendrimer N-acetyl-cysteine) in Severe Coronavirus Disease 2019 (COVID-19) Patients | United States | Y | N | Shelved drug or NME | OP-101 | Safety | Severe Cases |
| NCT04351243 | A Study to Assess the Efficacy and Safety of Gimsilumab in Subjects With Lung Injury or Acute Respiratory Distress Syndrome Secondary to COVID-19 (BREATHE) | United States | Y | N | Shelved drug or NME | Gimsilumab | Efficacy - Mortality | Severe Cases |
| NCT04355247 | Prophylactic Corticosteroid to Prevent COVID-19 Cytokine Storm | United States | N | N | Drug Repurposing | Methylprednisolone | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04405908 | SCB-2019 as COVID-19 Vaccine | China | Y | Y | Vaccine | ND:Vaccine | Safety | General population |
| NCT04353284 | Camostat Mesylate in COVID-19 Outpatients | United States | Y | N | Drug Repurposing | Camostat | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04389710 | Convalescent Plasma for the Treatment of COVID-19 | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Other | Severe Cases |
| NCT04494399 | IFN Beta-1b and Ribavirin for Covid-19 | Hong Kong | Y | N | Drug Repurposing | Interferon beta-1b, Ribavirin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04380402 | Atorvastatin as Adjunctive Therapy in COVID-19 | United States | Y | N | Drug Repurposing | Atorvastatin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04374903 | Hydroxychloroquine in Combination With Azithromycin or Sirolimus for Treating COVID-19 Patients | Jordan | Y | N | Drug Repurposing | Azithromycin, Sirolimus | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04386239 | Study on the Use of Sarilumab in Patients With COVID-19 Infection | Italy | N | N | Drug Repurposing | Sarilumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04354805 | Administration of Chlorpromazine as a Treatment for COVID-19 | Egypt | Y | N | Drug Repurposing | Chlorpromazine | Other | Mild/Moderate Cases, Severe Cases |
| NCT04351763 | Amiodarone or Verapamil in COVID-19 Hospitalized Patients With Symptoms | Poland | Y | Y | Drug Repurposing | Amiodarone, Verapamil | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04388527 | COVID-19 Convalescent Plasma for Mechanically Ventilated Population | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Safety | Severe Cases |
| NCT04340544 | Hydroxychloroquine for the Treatment of Mild COVID-19 Disease | Germany | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04487444 | Thymalfasin (Thymosin Alpha 1) to Treat COVID-19 Infection | United States | Y | N | Drug Repurposing | Thymalfasin | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04338828 | Nitric Oxide Inhalation Therapy for COVID-19 Infections in the ED | United States | Y | N | Drug Repurposing | Nitric Oxide | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04480424 | Study to Evaluate the Safety and Efficacy of High Dose Intravenous Immune Globulin (IVIG) Plus Standard Medical Treatment (SMT) Versus SMT Alone in Participants in Intensive Care Unit (ICU) With Coronavirus Disease (COVID-19) | United States | Y | N | Cell/Blood based | ND:Immunoglobulin | Efficacy - Mortality | Severe Cases |
| NCT04468087 | Antiviral Agents Against COVID-19 Infection | Brazil | Y | Y | Drug Repurposing | Atazanavir, Daclatasvir, Sofusbuvir | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04338958 | Ruxolitinib in Covid-19 Patients With Defined Hyperinflammation | Germany | N | N | Drug Repurposing | Ruxolitinib | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04356690 | Etoposide in Patients With COVID-19 Infection | United States | N | N | Drug Repurposing | Etoposide | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04472494 | Study of Abatacept in the Treatment of Hospitalized COVID-19 Participants With Respiratory Compromise | United States | Y | N | Drug Repurposing | Abatacept | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04415151 | Tofacitinib for Treatment of Moderate COVID-19 | United States | Y | N | Drug Repurposing | Tofacitinib | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04375124 | Treatment of Angiotensin Peptide (1-7) for COVID-19 | Turkey | N | N | Drug Repurposing | Angiotensin 1-7 | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04371952 | DYNAMIC Study (DoxycYcliNe AMbulatoIre COVID-19) | France | Y | N | Drug Repurposing | Doxycycline | Efficacy - Oxygen parameters | Mild/Moderate Cases |
| NCT04371822 | Efficacy of Sunlight Activated Synthetic Porphyrin in COVID-19 Infected Patients | Egypt | Y | Y | Shelved drug or NME | SnPP Protoporphyrin, Sulfonatoporphyrin | Efficacy - Infection rate (prevention) | Mild/Moderate Cases, Severe Cases |
| NCT04427098 | Enoxaparin in COVID-19 Moderate to Severe Hospitalized Patients | Iceland | N | N | Drug Repurposing | Enoxaparin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04438694 | Use of Convalescent Plasma for Treatment of Patients With COVID-19 Infection | Egypt | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Severe Cases |
| NCT04443725 | Efficacy and Safety of Anti HCV Drugs in the Treatment of COVID-19 | Egypt | Y | N | Drug Repurposing | Hydroxychloroquine, Sofosbuvir, Daclatasvir | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04366115 | Evaluating AVM0703 for Treatment of COVID-19 | United States | Y | N | Drug Repurposing, Shelved drug or NME | AVM0703 | Safety | Severe Cases |
| NCT04383002 | High Dose Inhaled Nitric Oxide for COVID-19 (ICU Patients) | Canada | Y | N | Drug Repurposing | Nitric Oxide | Efficacy - Viral load | Severe Cases |
| NCT04410328 | Aggrenox To Treat Acute Covid-19 | United States | Y | N | Drug Repurposing | Aspirin/dipyridamole | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04445935 | Anticoagulation in Patients Suffering From COVID-19 Disease The ANTI-CO Trial | Qatar | Y | N | Drug Repurposing | Bivalirudin | Efficacy - Oxygen parameters | Severe Cases |
| NCT04343092 | Efficacy of Ivermectin as Add on Therapy in COVID19 Patients | Iraq | Y | N | Drug Repurposing | Ivermectin | Efficacy - Other efficacy | Severe Cases |
| NCT04338074 | TXA and Corona Virus 2019 (COVID19) in Outpatients | United States | Y | N | Drug Repurposing | Tranexamic acid | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04494724 | Clazakizumab vs. Placebo - COVID-19 Infection | United States | Y | N | Shelved drug or NME | Clazakizumab | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04366050 | Ramipril for the Treatment of COVID-19 | United States | Y | N | Drug Repurposing | Ramipril | Efficacy - Mortality | Severe Cases |
| NCT04438850 | COVidIVERmectin: Ivermectin for Treatment of Covid-19 | Italy | Y | N | Drug Repurposing | Ivermectin | Safety | Mild/Moderate Cases |
| NCT04338126 | Tranexamic Acid (TXA) and Corona Virus 2019 (COVID19) in Inpatients | United States | Y | N | Drug Repurposing | Tranexamic acid | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04355026 | Use of Bromhexine and Hydroxychloroquine for Treatment of COVID-19 Pneumonia | Slovenia | Y | N | Drug Repurposing | Bromhexine | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04341675 | Sirolimus Treatment in Hospitalized Patients With COVID-19 Pneumonia | United States | Y | N | Drug Repurposing | Sirolimus | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04444271 | Mesenchymal Stem Cell Infusion for COVID-19 Infection | Pakistan | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04469114 | Tofacitinib in Hospitalized Patients With COVID-19 Pneumonia | Brazil | Y | N | Drug Repurposing | Tofacitinib | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04446104 | A Preventive Treatment for Migrant Workers at High-risk of Covid-19 | Singapore | Y | Y | Drug Repurposing | Hydroxychloroquine, Ivermectin, Zinc, Povidone-Iodine | Efficacy - Oxygen parameters | Exposed individuals (family members, HCP) |
| NCT04329923 | The PATCH Trial (Prevention And Treatment of COVID-19 With Hydroxychloroquine) | United States | Y | Y | Drug Repurposing | Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04394416 | Trial of Imatinib for Hospitalized Adults With COVID-19 | United States | Y | N | Drug Repurposing | Imatinib | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04374461 | A Study of N-acetylcysteine in Patients With COVID-19 Infection | United States | N | N | Drug Repurposing | N-acetylcysteine | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04397757 | COVID-19 Convalescent Plasma for the Treatment of Hospitalized Patients With Pneumonia Caused by SARS-CoV-2. | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04361643 | Low-dose Lenalidomide for Non-severe COVID-19 Treatment Trial | Spain | Y | N | Drug Repurposing | Lenalidomide | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04455243 | Inflammatory Regulation Effect of NAC on COVID-19 Treatment | Saudi Arabia | Y | N | Drug Repurposing | N-acetylcysteine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04333628 | Chloroquine for Mild Symptomatic and Asymptomatic COVID-19 | Israel | Y | Y | Drug Repurposing | Chloroquine | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04463602 | Desidustat in the Management of COVID-19 Patients | Mexico | Y | N | Shelved drug or NME | Desidustat | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04372628 | Trial of Early Therapies During Non-hospitalized Outpatient Window for COVID-19 | United States | Y | N | Drug Repurposing | Lopinavir/ritonavir | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04429763 | Safety and Efficacy of Mesenchymal Stem Cells in the Management of Severe COVID-19 Pneumonia | Colombia | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Other efficacy | Severe Cases |
| NCT04304053 | Treatment of COVID-19 Cases and Chemoprophylaxis of Contacts as Prevention | Spain | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP), Mild/Moderate Cases |
| NCT04421027 | A Study of Baricitinib (LY3009104) in Participants With COVID-19 | United States | Y | N | Drug Repurposing | Baricitinib | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04355143 | Colchicine to Reduce Cardiac Injury in COVID-19 (COLHEART-19) | United States | Y | N | Drug Repurposing | Colchicine | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04451174 | Early Use of Corticosteroids in Hospitalized Patients With Moderate COVID19 Pneumonia | Chile | Y | N | Drug Repurposing | Prednisone | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04362085 | Coagulopathy of COVID-19: A Pragmatic Randomized Controlled Trial of Therapeutic Anticoagulation Versus Standard Care | Canada | Y | N | Drug Repurposing | Enoxaparin, Dalteparin, Tinzaparin, Heparin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04351620 | High-dose Hydroxychloroquine for the Treatment of Ambulatory Patients With Mild COVID-19 | United States | N | N | Drug Repurposing | Hydroxychloroquine | Safety | Mild/Moderate Cases |
| NCT04390464 | mulTi-Arm Therapeutic Study in Pre-ICu Patients Admitted With Covid-19 - Repurposed Drugs (TACTIC-R) | United Kingdom | Y | Y | Drug Repurposing | Ravulizumab, Baricitinib | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04480138 | Pegylated Interferon - a2b With SARSCoV- 2 (COVID-19) | Mexico | Y | N | Drug Repurposing | Interferon alpha-2b | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04363216 | Pharmacologic Ascorbic Acid as an Activator of Lymphocyte Signaling for COVID-19 Treatment | United States | Y | N | Drug Repurposing | Ascorbic Acid | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04339426 | Atovaquone and Azithromycin Combination for Confirmed COVID-19 Infection | United States | N | N | Drug Repurposing | Atovaquone, Azithromycin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04334382 | Hydroxychloroquine vs. Azithromycin for Outpatients in Utah With COVID-19 | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04437693 | Post Exposure Prophylaxis in Healthcare Workers Exposed to COVID-19 Patients | Qatar | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04438057 | Evaluating the Efficacy of Convalescent Plasma in Symptomatic Outpatients Infected With COVID-19 | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04350320 | Trial to Study the Benefit of Colchicine in Patients With COVID-19 | Spain | Y | N | Drug Repurposing | Colchicine | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04342182 | Convalescent Plasma as Therapy for Covid-19 Severe SARS-CoV-2 Disease (CONCOVID Study) | Netherlands | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| NCT04343001 | Coronavirus Response - Active Support for Hospitalised Covid-19 Patients | United Kingdom | Y | Y | Drug Repurposing | Aspirin, Losartan, Simvastatin | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04463420 | Safety and Efficacy of PHR 160 Spray on the Outcomes of Patients With COVID-19 | Iran | Y | N | Shelved drug or NME | PHR160 Spray | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04410159 | Povidone-Iodine Vs Essential Oil Vs Tap Water Gargling For COVID-19 Patients | Malaysia | Y | Y | Shelved drug or NME | Povidone-Iodine | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04357990 | Kerecis Oral and Nasal Spray for Treating the Symptoms of COVID-19 | Iceland | Y | N | Shelved drug or NME | Omega3 Viruxide | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04356534 | Convalescent Plasma Trial in COVID -19 Patients | Bahrain | Y | N | Cell/Blood based | ND:Covalescent plasma | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04393311 | Ulinastatin for the Treatment of COVID-19 in Hospitalized Patients | United States | Y | N | Shelved drug or NME | Ulinastatin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04348500 | Clazakizumab (Anti-IL- 6 Monoclonal) Compared to Placebo for COVID19 Disease | United States | Y | N | Shelved drug or NME | Clazakizumab | Efficacy - Oxygen parameters | Severe Cases |
| NCT04392414 | Hyperimmune Convalescent Plasma in Moderate and Severe COVID-19 Disease | Russian Federation | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04442191 | Convalescent Plasma as a Possible Treatment for COVID-19 | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Oxygen parameters | Mild/Moderate Cases |
| NCT04362189 | Efficacy and Safety Study of Allogeneic HB-adMSCs for the Treatment of COVID-19 | United States | Y | Y | Cell/Blood based | ND:Stem cells | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04409522 | Evaluation of Therapeutic Effects of Melatonin by Inhibition of NLRP3 Inflammasome in COVID19 Patients | Iran | Y | N | Drug Repurposing | Melatonin | Other | Mild/Moderate Cases, Severe Cases |
| NCT04360980 | The Effects of Standard Protocol With or Without Colchicine in Covid-19 Infection | Iran | Y | N | Drug Repurposing | Colchicine | Other | Mild/Moderate Cases |
| NCT04335136 | Recombinant Human Angiotensin-converting Enzyme 2 (rhACE2) as a Treatment for Patients With COVID-19 | Denmark | Y | N | Shelved drug or NME | Recombinant Human Angiotensin-converting Enzyme 2 | Efficacy - Other efficacy | Severe Cases |
| NCT04391127 | Hydroxychloroquine and Ivermectin for the Treatment of COVID-19 Infection | Mexico | Y | N | Drug Repurposing | Ivermectin, Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04359537 | Efficacy of Various Doses of Hydroxychloroquine in Pre-Exposure Prophylaxis for COVID 19 | Pakistan | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04457726 | Part Two of Novel Adoptive Cellular Therapy With SARS-CoV-2 Specific T Cells in Patients With Severe COVID-19 | Singapore | N | N | Cell/Blood based | ND:T-cells | Safety | Severe Cases |
| NCT04492254 | Early Prophylactic Low-molecular-weight Heparin (LMWH) in Symptomatic COVID-19 Positive Patients | United Kingdom | Y | N | Drug Repurposing | Enoxaparin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04276896 | Immunity and Safety of Covid-19 Synthetic Minigene Vaccine | China | N | N | Cell/Blood based | ND:T-cells | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04370236 | XPro1595 for the Treatment of Pulmonary Complications From COVID-19 | United States | Y | N | Drug Repurposing | XPro1595 | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04359511 | Efficacy and Safety of Corticosteroids in Oxygen-dependent Patients With COVID-19 Pneumonia | France | Y | N | Drug Repurposing | Prednisone, Hydrocortisone | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04331795 | Tocilizumab to Prevent Clinical Decompensation in Hospitalized, Non-critically Ill Patients With COVID-19 Pneumonitis | United States | N | Y | Drug Repurposing | Tocilizumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04456452 | Study of Ampion for the Treatment of Adult COVID-19 Patients Requiring Oxygen Supplementation | United States | Y | N | Drug Repurposing | Ampion | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04333225 | Hydroxychloroquine in the Prevention of COVID-19 Infection in Healthcare Workers | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04405843 | Efficacy of Ivermectin in Adult Patients With Early Stages of COVID-19 | Colombia | Y | N | Drug Repurposing | Ivermectin | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04351724 | Austrian CoronaVirus Adaptive Clinical Trial (COVID-19) | Austria | Y | Y | Drug Repurposing, Shelved drug or NME | Hydroxychloroquine, Chloroquine, Lopinavir/ritonavir, Rivaroxaban, Candesartan, Clazakizumab | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04393246 | mulTi-Arm Therapeutic Study in Pre-ICu Patients Admitted With Covid-19 - Experimental Drugs and Mechanisms | United Kingdom | Y | N | Shelved drug or NME | EDP1815, Dapagliflozin, Ambrisentan | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04344444 | Treatment in Patients With Suspected or Confirmed COVID-19 With Early Moderate or Severe Disease | United States | Y | Y | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04429867 | Hydroxychloroquine Use in Hospitalized Patients With COVID-19: Impact on Progression to Severe or Critical Disease | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04377659 | Tocilizumab for Prevention of Respiratory Failure in Patients With Severe COVID-19 Infection | United States | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04425772 | A Clinical Trial for Azvudine in the Treatment of Novel Coronavirus Pneumonia (COVID-19) | United States | Y | N | Drug Repurposing | Azvudine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04352465 | Efficacy and Safety of MTX-loaded Nanoparticles to Treat Severe COVID-19 Patients | Brazil | N | N | Shelved drug or NME | Methotrexate nano-formulation | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04435314 | Efficacy and Safety of Nitazoxanide for Post Exposure Prophylaxis of COVID-19 | Brazil | Y | N | Drug Repurposing | Nitazoxanide | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04352751 | Experimental Use of Convalescent Plasma for Passive Immunization in Current COVID-19 Pandemic in Pakistan in 2020 | Pakistan | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04389801 | Use of Desferal for Prevention of ARDS in Hospitalised Cases Documented With Covid 19 Infection | Egypt | Y | N | Drug Repurposing | Deferoxamine | Efficacy - Mortality | Mild/Moderate Cases |
| NCT04427501 | A Study of LY3819253 (LY-CoV555) in Participants With Mild to Moderate COVID-19 Illness | United States | Y | N | Shelved drug or NME | LY3819253 | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04486313 | Trial to Evaluate Efficacy and Safety of Nitazoxanide in the Treatment of Mild or Moderate COVID-19 | United States | Y | N | Drug Repurposing | Nitazoxanide, ND:Dietary supplement | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04374487 | Assess the Safety and Efficacy of Convalescent Plasma to Limit COVID-19 Associated Complications | India | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04328441 | Reducing Health Care Workers Absenteeism in Covid-19 Pandemic Through BCG Vaccine | Netherlands | Y | N | Drug Repurposing, Vaccine | BCG Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04432766 | A Phase 1 Study to Assess BAT2020 in Hospitalized Patients Infected With SARS-CoV-2 (COVID-19) | China | Y | N | Shelved drug or NME | BAT2020 | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04429711 | Ivermectin vs. Placebo for the Treatment of Patients With Mild to Moderate COVID-19 | Israel | Y | N | Drug Repurposing | Ivermectin | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04307693 | Comparison of Lopinavir/Ritonavir or Hydroxychloroquine in Patients With Mild Coronavirus Disease (COVID-19) | Korea, Republic of | Y | Y | Drug Repurposing | Hydroxychloroquine, Lopinavir/ritonavir | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04329650 | Efficacy and Safety of Siltuximab vs. Corticosteroids in Hospitalized Patients With COVID-19 Pneumonia | Spain | Y | N | Drug Repurposing | Siltuximab, Methylprednisolone | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04468139 | The Study of Quadruple Therapy Zinc, Quercetin, Bromelain and Vitamin C on the Clinical Outcomes of Patients Infected With COVID-19 | Saudi Arabia | N | N | Drug Repurposing, Other | Ascorbic acid, ND:Quercetin/bromelain, Zinc | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04385849 | Study of the Safety of Therapeutic Treatment With an Immunomodulatory Agent (N-803) in Adults With COVID-19 | United States | Y | N | Shelved drug or NME | N-803 | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04454398 | Study to Evaluate STI-1499 (COVI-GUARD) in Hospitalized Patients With COVID-19 | United States | Y | N | Cell/Blood based | STI-1499 | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04363814 | Clinical Impact of BACTEK-R in Subject With Mild Pneumonia Due to COVID-19 Infection | United States | Y | N | Shelved drug or NME | Bactek-R | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04345692 | A Randomized Controlled Clinical Trial: Hydroxychloroquine for the Treatment of COVID-19 in Hospitalized Patients | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - WHO scale | Severe Cases |
| NCT04364763 | A Study to Evaluate the Effect of RBT-9 on Progression of COVID-19 in High-Risk Individuals (The PREVENT Study) | United States | N | N | Shelved drug or NME | RBT-9 | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04397562 | A Clinical Trial of the Efficacy and Safety of Levilimab (BCD-089) in Patients With Severe COVID-19 | Russian Federation | Y | N | Shelved drug or NME | Levilimab | Efficacy - Mortality | Severe Cases |
| NCT04331899 | Single-Blind Study of a Single Dose of Peginterferon Lambda-1a Compared With Placebo in Outpatients With Mild COVID-19 | United States | Y | N | Shelved drug or NME | Peg-Interferon lambda-1a | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04397718 | Hormonal Intervention for the Treatment in Veterans With COVID-19 Requiring Hospitalization | United States | Y | N | Drug Repurposing | Degarelix | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04362332 | Chloroquine, Hydroxychloroquine or Only Supportive Care in Patients AdmItted With Moderate to Severe COVID-19 | Netherlands | Y | Y | Drug Repurposing | Chloroquine, Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04441398 | Efficacy and Safety of Nitazoxanide 600 mg to Treat Mild Ambulatory COVID-19 Patients | Brazil | Y | N | Drug Repurposing | Nitazoxanide | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04486508 | Intermediate-dose vs Standard Prophylactic Anticoagulation and Statin vs Placebo in ICU Patients With COVID-19 | Iran | Y | Y | Drug Repurposing | Enoxaparin, Atorvastatin | Efficacy - Other efficacy | Severe Cases |
| NCT04434131 | Treatment With Investigational Convalescent Plasma and Measure Antibody Levels in Patients Hospitalized With COVID-19 | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04331834 | Pre-Exposure Prophylaxis With Hydroxychloroquine for High-Risk Healthcare Workers During the COVID-19 Pandemic | Spain | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04331470 | Evaluation of Efficacy of Levamisole and Formoterol+Budesonide in Treatment of COVID-19 | Iran | Y | N | Drug Repurposing | Levamisole, Budesonide/formoterol | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04440007 | Study of the Efficacy and Safety of STI-5656 (Abivertinib Maleate) With SOC Versus SOC in Subjects With COVID-19 | United States | Y | N | Shelved drug or NME | Abivertinib | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04423861 | Efficacy and Safety of Nitazoxanide 600 mg TID for the Treatment of Hospitalized Patients With COVID-19 | Brazil | Y | N | Drug Repurposing | Nitazoxanide | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04386252 | Phase Ib-II Trial of Dendritic Cell Vaccine to Prevent COVID-19 in Adults | United States | Y | N | Vaccine | ND:Vaccine | Safety | Exposed individuals (family members, HCP) |
| NCT04356677 | Study to Evaluate the Safety and Efficacy of VIRAZOLE® in Hospitalized Adult Participants With Respiratory Distress Due to COVID-19 | United States | N | N | Drug Repurposing | Ribavirin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04313023 | The Use PUL-042 to Reduce the Infection Rate and Progression to COVID-19 in Adults Exposed to SARS-CoV-2 | United States | Y | N | Shelved drug or NME | PUL-042 | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04409327 | Phase 3 Study to Determine if RTB101 Reduces the Severity of COVID-19 in Older Adults Residing in Nursing Homes | United States | Y | N | Shelved drug or NME | RTB101 | Efficacy - Other efficacy | Other |
| NCT04336254 | Safety and Efficacy Study of Allogeneic Human Dental Pulp Mesenchymal Stem Cells to Treat Severe COVID-19 Patients | China | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04292899 | Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Severe Coronavirus Disease (COVID-19) | United States | N | N | Shelved drug or NME | Remdesivir | Efficacy - Oxygen parameters | Severe Cases |
| NCT04416139 | Mesenchymal Stem Cell for Acute Respiratory Distress Syndrome Due for COVID-19 | Mexico | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04332094 | Clinical Trial of Combined Use of Hydroxychloroquine, Azithromycin, and Tocilizumab for the Treatment of COVID-19 | Brazil | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Mortality | Mild/Moderate Cases |
| NCT04456595 | Clinical Trial of Efficacy and Safety of Sinovac's Adsorbed COVID-19 (Inactivated) Vaccine in Healthcare Professionals | Brazil | Y | N | Vaccine | ND:Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04466280 | Efficacy and Safety of Mucoadhesive Sustained Release, Mucodentol, in Comparison With Hydroxychloroquine to Prevent COVID-19 | Iran | Y | N | Drug Repurposing, Shelved drug or NME | Hydroxychloroquine, Mucodentol | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04312997 | The Use of PUL-042 Inhalation Solution to Reduce the Severity of COVID-19 in Adults Positive for SARS-CoV-2 Infection | United States | Y | N | Shelved drug or NME | PUL-042 | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04385264 | #StayHome: Early Hydroxychloroquine to Reduce Secondary Hospitalisation and Household Transmission in COVID-19 | Switzerland | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04425460 | A Multi-center,Randomized,Double-blind,Placebo-controlled,Phase 3 Study Evaluating Favipiravir in Treatment of COVID19 | China | Y | N | Drug Repurposing | Favipiravir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04370782 | Hydroxychloroquine and Zinc With Either Azithromycin or Doxycycline for Treatment of COVID-19 in Outpatient Setting | United States | Y | N | Drug Repurposing | Hydroxychloroquine, Azithromycin, Zinc, Doxycycline | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04334980 | Evaluating the Safety, Tolerability and Immunogenicity of bacTRL-Spike Vaccine for Prevention of COVID-19 | Canada | Y | Y | Vaccine | bacTRL-Spike vaccine | Safety | Other |
| NCT04478019 | SHIELD Study: Using Naso-oropharyngeal Antiseptic Decolonization to Reduce COVID-19 Viral Shedding | United States | Y | N | Shelved drug or NME | Povidone-Iodine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04348409 | Efficacy and Safety of Nitazoxanide for the Treatment of Hospitalized Patients With Moderate COVID-19 | Brazil | Y | N | Drug Repurposing | Nitazoxanide | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04377711 | A Study of the Safety and Efficacy of Ciclesonide in the Treatment of Non-hospitalized COVID-19 Patients | Switzerland | Y | N | Drug Repurposing | Ciclesonide | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04353518 | Clinical Trial to Evaluate Mycobacterium w in Preventing COVID-19 in Subjects at Risk of Getting Infected With COVID-19 | India | Y | N | Shelved drug or NME | ND:Mycobacterium | Efficacy - Infection rate (prevention) | General population |
| NCT04429334 | Safety, Tolerability and Efficacy of Nangibotide in Mechanically Ventilated Patients With COVID-19 and Features of Systemic Inflammation | France | Y | N | Shelved drug or NME | Nangibotide | Safety | Severe Cases |
| NCT04328493 | The Vietnam Chloroquine Treatment on COVID-19 | Viet Nam | Y | N | Drug Repurposing | Chloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04360551 | Pilot Clinical Trial of the Safety and Efficacy of Telmisartan for the Mitigation of Pulmonary and Cardiac Complications in COVID-19 Patients | United States | Y | N | Drug Repurposing | Telmisartan | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04343989 | A Randomized Placebo-controlled Safety and Dose-finding Study for the Use of the IL-6 Inhibitor Clazakizumab in Patients With Life-threatening COVID-19 Infection | United States | Y | Y | Shelved drug or NME | Clazakizumab | Safety | Severe Cases |
| NCT04348474 | Efficacy and Safety of Hydroxychloroquine and Azithromycin for the Treatment of Ambulatory Patients With Mild COVID-19 | Brazil | N | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04470531 | Role of Co-trimoxazole in Severe COVID-19 Patients | Bangladesh | Y | N | Drug Repurposing | Trimethoprim/sulfamethoxazole | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04488575 | Safety and Efficacy of EDP1815 in the Treatment of Patients Hospitalized With COVID-19 Infection | United States | Y | N | Shelved drug or NME | EDP1815 | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04358809 | Clinical Trial of Mycobacterium w in COVID-19 Positive Patients, Hospitalized But Not Critically Ill | India | Y | N | Vaccine | ND:Mycobacterium | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04492358 | Treatment for Moderate/Severe COVID-19 in a Fragile and Vulnerable Population, Admitted to a Geriatric Hospital Unit or in a Transicional Care Center | Spain | Y | N | Drug Repurposing | Colchicine, Prednisone | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04348435 | A Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Determine the Safety and Efficacy of Hope Biosciences Allogeneic Mesenchymal Stem Cell Therapy (HB-adMSCs) to Provide Protection Against COVID-19 | United States | Y | Y | Cell/Blood based | ND:Stem cells | Efficacy - Other efficacy | Exposed individuals (family members, HCP) |
| NCT04329572 | Efficacy and Safety of Hydroxychloroquine and Azithromycin for the Treatment of Hospitalized Patients With Moderate to Severe COVID-19 | Brazil | N | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT03348670 | Follow NCT03305341 - Proof-of-Concept - COVID-19 AP TP Vaccine | United States | N | N | Vaccine | ND:Vaccine | Other | Mild/Moderate Cases |
| NCT04292730 | Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment | United States | Y | Y | Shelved drug or NME | Remdesivir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04431453 | Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Remdesivir (GS-5734™) in Participants From Birth to < 18 Years of Age With Coronavirus Disease 2019 (COVID-19) | United States | N | N | Shelved drug or NME | Remdesivir | Safety | Other |
| NCT04358549 | Study of the Use of Favipiravir in Hospitalized Subjects With COVID-19 | United States | Y | N | Drug Repurposing | Favipiravir | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04482699 | RAPA-501-Allo Off-the-Shelf Therapy of COVID-19 | United States | Y | Y | Cell/Blood based | ND:MSC | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04481685 | A Trial of Aclaris Therapeutics, Inc. (ATI)-450 in Patients With Moderate-severe Novel Coronavirus Disease 2019 (COVID-19) | United States | Y | N | Shelved drug or NME | ATI-450 | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT03305341 | Discovery Stage (Proof-of-concept) COVID-19 Antigen Presentation Therapeutic Vaccine | United States | N | N | Vaccine | ND:Vaccine | Other | Mild/Moderate Cases |
| NCT04428021 | Standard or Convalescent Plasma in Patients With Recent Onset of COVID-19 Respiratory Failure | Italy | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04346615 | Safety and Efficacy Trial of Zavegepant* Intranasal for Hospitalized Patients With COVID-19 Requiring Supplemental Oxygen | United States | Y | N | Shelved drug or NME | Vazegepant | Safety | Severe Cases |
| NCT04428268 | Chloroquine + Losartan Compared to Chloroquine Alone for the Treatment of COVID-19 Pneumonia | Mexico | Y | N | Drug Repurposing | Losartan | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04360824 | Covid-19 Associated Coagulopathy | United States | Y | N | Drug Repurposing | Enoxaparin | Efficacy - Mortality | General population, Mild/Moderate Cases |
| NCT04463264 | Efficacy and Safety Study of Nitazoxanide (NTX) in the Treatment of Patients With SARS-CoV-2 Virus Infection (COVID-19) | Argentina | Y | N | Drug Repurposing | Nitazoxanide | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04368728 | Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Adults | United States | Y | Y | Shelved drug or NME | BNT162b1, BNT162b2 | Safety | General population |
| NCT04415060 | SedAting With Volatile Anesthetics Critically Ill COVID-19 Patients in ICU: Effects On Ventilatory Parameters And Survival | Canada | Y | N | Drug Repurposing | Isoflurane, Sevoflurane | Efficacy - Mortality | Severe Cases |
| NCT04349410 | The Fleming [FMTVDM] Directed CoVid-19 Treatment Protocol | United States | Y | Y | Drug Repurposing, Shelved drug or NME, Cell/Blood based | Hydroxychloroquine, Azithromycin, Doxycycline, Clindamycin, Primaquine, Remdesivir, Tocilizumab, Methylprednisolone, Losartan, ND:Convalescent plasma, Interferon alpha-2b | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04387760 | Favipiravir vs Hydroxychloroquine in COVID -19 | Bahrain | Y | Y | Drug Repurposing | Hydroxychloroquine, Favipiravir | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04460651 | PREPARE-IT. Prevention of COVID19 With EPA in Healthcare Providers at Risk - Intervention Trial | Argentina | Y | N | Drug Repurposing | Icosapent ethyl | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04364802 | COVID-19: Povidone-Iodine Intranasal Prophylaxis in Front-line Healthcare Personnel and Inpatients | United States | N | N | Shelved drug or NME | Povidone-Iodine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04315896 | Hydroxychloroquine Treatment for Severe COVID-19 Pulmonary Infection (HYDRA Trial) | Mexico | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Mortality | Severe Cases |
| NCT04408209 | Convalescent Plasma for the Treatment of Patients With Severe COVID-19 Infection | Greece | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| NCT04323514 | Use of Ascorbic Acid in Patients With COVID 19 | Italy | N | N | Drug Repurposing | Ascorbic acid | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04273646 | Study of Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Severe COVID-19 | China | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04381858 | Convalescent Plasma vs Human Immunoglobulin to Treat COVID-19 Pneumonia | Mexico | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Severe Cases |
| NCT04494646 | BARCONA: A Study of Effects of Bardoxolone Methyl in Participants With SARS-Corona Virus-2 (COVID-19) | United States | Y | N | Shelved drug or NME | Bardoxolone | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04461925 | Treatment of Coronavirus COVID-19 Pneumonia (Pathogen SARS-CoV-2) With Cryopreserved Allogeneic P_MMSCs and UC-MMSCs | Ukraine | N | N | Cell/Blood based | ND:MSC | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04477083 | Development and Validation of "Ready-to-Use" Inhalable Forms of Hydroxychloroquine for Treatment of COVID-19 | Egypt | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04362137 | Phase 3 Randomized, Double-blind, Placebo-controlled Multi-center Study to Assess the Efficacy and Safety of Ruxolitinib in Patients With COVID-19 Associated Cytokine Storm (RUXCOVID) | United States | Y | N | Drug Repurposing | Ruxolitinib | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04305106 | Bevacizumab in Severe or Critically Severe Patients With COVID-19 Pneumonia-RCT | China | Y | N | Drug Repurposing | Bevacizumab | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04315987 | NestaCell® Mesenchymal Stem Cell to Treat Patients With Severe COVID-19 Pneumonia | Brazil | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Other efficacy | Severe Cases |
| NCT04460105 | Lanadelumab in Participants Hospitalized With COVID-19 Pneumonia | United States | Y | N | Drug Repurposing | Lanadelumab | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04354870 | COVID-19 PrEP HCW HCQ Study | United States | N | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04244591 | Glucocorticoid Therapy for COVID-19 Critically Ill Patients With Severe Acute Respiratory Failure | China | Y | N | Drug Repurposing | Methylprednisolone | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04342663 | A Double-blind, Placebo-controlled Clinical Trial of Fluvoxamine for Symptomatic Individuals With COVID-19 Infection | United States | Y | N | Drug Repurposing | Fluvoxamine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04325061 | Efficacy of Dexamethasone Treatment for Patients With ARDS Caused by COVID-19 | Spain | Y | N | Drug Repurposing | Dexamethasone | Efficacy - Mortality | Severe Cases |
| NCT04320615 | A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia | United States | Y | N | Drug Repurposing | Tocilizumab | Efficacy - WHO scale | Severe Cases |
| NCT04344730 | Dexamethasone and Oxygen Support Strategies in ICU Patients With Covid-19 Pneumonia | France | Y | Y | Drug Repurposing, Other | Dexamethasone, ND:Procedure | Efficacy - Mortality | Severe Cases |
| NCT04372186 | A Study to Evaluate the Efficacy and Safety of Tocilizumab in Hospitalized Participants With COVID-19 Pneumonia | United States | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04407130 | Efficacy and Safety of Ivermectin and Doxycycline in Combination or IVE Alone in Patients With COVID-19 Infection. | Bangladesh | Y | Y | Drug Repurposing | Ivermectin, Doxycycline | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04386616 | A Study to Evaluate the Safety and Efficacy of MSTT1041A (Astegolimab) or UTTR1147A in Patients With Severe COVID-19 Pneumonia | United States | Y | N | Shelved drug or NME | Astegolimab | Efficacy - WHO scale | Severe Cases |
| NCT04332107 | Azithromycin for COVID-19 Treatment in Outpatients Nationwide | United States | Y | N | Drug Repurposing | Azithromycin | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04363736 | A Study to Investigate Intravenous Tocilizumab in Participants With Moderate to Severe COVID-19 Pneumonia | United States | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04363060 | Azithromycin+Amoxicillin/Clavulanate vs Amoxicillin/Clavulanate in COVID19 Patients With Pneumonia in Non-intensive Unit | France | Y | N | Drug Repurposing | Azithromycin, Amoxicillin/clavulanate | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04371640 | Sirolimus in COVID-19 Phase 1 | United States | Y | N | Drug Repurposing | Sirolimus | Efficacy - Viral load | Severe Cases |
| NCT04455815 | A Trial Looking at the Use of Camostat to Reduce Progression of Symptoms of Coronavirus (COVID-19) in People Who Have Tested Positive But Are Able to Stay at Home | United Kingdom | Y | N | Drug Repurposing | Camostat | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04417257 | Study of LAU-7b for the Treatment of COVID-19 Disease in Adults | Canada | Y | N | Shelved drug or NME | LAU-7b | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04273529 | The Efficacy and Safety of Thalidomide in the Adjuvant Treatment of Moderate New Coronavirus (COVID-19) Pneumonia | China | Y | N | Drug Repurposing | Thalidomide | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04273581 | The Efficacy and Safety of Thalidomide Combined With Low-dose Hormones in the Treatment of Severe COVID-19 | China | Y | N | Drug Repurposing | Thalidomide | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04409262 | A Study to Evaluate the Efficacy and Safety of Remdesivir Plus Tocilizumab Compared With Remdesivir Plus Placebo in Hospitalized Participants With Severe COVID-19 Pneumonia | United States | Y | N | Drug Repurposing | Tocilizumab | Efficacy - WHO scale | Severe Cases |
| NCT04335201 | Defibrotide in COVID-19 Pneumonia | Italy | N | N | Drug Repurposing | Defibrotide | Efficacy - Other efficacy | Severe Cases |
| NCT04379336 | BCG Vaccination for Healthcare Workers in COVID-19 Pandemic | South Africa | Y | N | Drug Repurposing | BCG Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04283461 | Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19) | United States | N | Y | Vaccine | ND:Vaccine | Safety | General population |
| NCT04374032 | Clinical Trial to Evaluate the Efficacy and Safety of an Immunomodulatory Therapy for the Treatment of Patients With Moderate to Severe COVID-19 Infection | Bosnia and Herzegovina | Y | N | Drug Repurposing | Metenkefalin, Tridecactide | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04359680 | Trial to Evaluate the Efficacy and Safety of Nitazoxanide (NTZ) for Post Exposure Prophylaxis of COVID-19 and Other Viral Respiratory Illnesses (VRI) in Healthcare Workers | United States | Y | N | Drug Repurposing | Nitazoxanide | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04334044 | Treatment of SARS Caused by COVID-19 With Ruxolitinib | Mexico | N | N | Drug Repurposing | Ruxolitinib | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04342221 | Hydroxychloroquine for COVID-19 | Germany | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04362124 | Performance Evaluation of BCG Vaccination in Healthcare Personnel to Reduce the Severity of SARS-COV-2 Infection | Colombia | Y | N | Drug Repurposing | BCG Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04365127 | Progesterone for the Treatment of COVID-19 in Hospitalized Men | United States | Y | N | Drug Repurposing | Progesterone | Efficacy - WHO scale | Severe Cases, Other |
| NCT04382053 | Study of Efficacy and Safety of DV890 in Patients With COVID-19 Pneumonia | United States | Y | N | Shelved drug or NME | DFV890 | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04353271 | Trial of Hydroxychloroquine In Covid-19 Kinetics | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04343248 | Trial to Evaluate the Efficacy and Safety of Nitazoxanide (NTZ) for Post-Exposure Prophylaxis of COVID-19 and Other Viral Respiratory Illnesses in Elderly Residents of Long-Term Care Facilities (LTCF) | United States | Y | N | Drug Repurposing | Nitazoxanide | Efficacy - Infection rate (prevention) | Other |
| NCT04372017 | Hydroxychloroquine as Post-Exposure Prophylaxis Against COVID-19 Infection | United States | Y | Y | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04449380 | Clinical Study for the Treatment With Interferon-ß-1a (IFNß-1a) of COVID-19 Patients | Italy | Y | N | Drug Repurposing | Interferon beta-1a | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04363203 | VA Remote and Equitable Access to COVID-19 Healthcare Delivery (VA-REACH TRIAL) | United States | Y | Y | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Other |
| NCT04341207 | Epidemiology of SARS-CoV-2 and Mortality to Covid19 Disease in French Cancer Patients | France | N | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04469179 | Safety, Tolerability, and Pharmacokinetics of SAB-185 in Ambulatory Participants With COVID-19 | United States | Y | Y | Shelved drug or NME | SAB-185 | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04252664 | A Trial of Remdesivir in Adults With Mild and Moderate COVID-19 | China | Y | N | Shelved drug or NME | Remdesivir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04413838 | Efficiency and Security of NIVOLUMAB Therapy in Obese Individuals With COVID-19(COrona VIrus Disease) Infection | France | Y | N | Drug Repurposing | Nivolumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases, Other |
| NCT04446065 | Protection of Health Workers Against COVID-19 | Chile | Y | N | Shelved drug or NME | Previfenon | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04405921 | Hydroxychloroquine, Azithromycin in the Treatment of Covid-19 | Tunisia | N | N | Drug Repurposing | Azithromycin, Hydroxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04346628 | Oral Favipiravir Compared to Placebo in Subjects With Mild COVID-19 | United States | Y | N | Drug Repurposing | Favipiravir | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04377646 | A Study of Hydroxychloroquine and Zinc in the Prevention of COVID-19 Infection in Military Healthcare Workers | Tunisia | Y | N | Drug Repurposing | Hydroxychloroquine, Zinc | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP), Other |
| NCT04382651 | Study of Efficacy and Safety of MAS825 in Patients With COVID-19 | United States | Y | N | Shelved drug or NME | MAS825 | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04362813 | Study of Efficacy and Safety of Canakinumab Treatment for CRS in Participants With COVID-19-induced Pneumonia | United States | Y | N | Drug Repurposing | Canakinumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04452643 | Efficacy of BACMUNE (MV130) in the Prevention of Disease Due to COVID-19 Infection in Healthcare Personnel | Mexico | Y | N | Shelved drug or NME | MV130 | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04494984 | A Study to Investigate the Pharmacokinetics, Efficacy and Safety of INM005 in Patients With COVID-19. | Argentina | Y | N | Shelved drug or NME | INM005 | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04470297 | Melatonin Agonist on Hospitalized Patients With Confirmed or Suspected COVID-19 | Brazil | Y | N | Drug Repurposing | Ramelteon | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04358068 | Evaluating the Efficacy of Hydroxychloroquine and Azithromycin to Prevent Hospitalization or Death in Persons With COVID-19 | United States | Y | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04437875 | An Open Study of the Safety, Tolerability and Immunogenicity of "Gam-COVID-Vac Lyo" Vaccine Against COVID-19 | Russian Federation | N | N | Vaccine | ND:Gam-COVID-Vac Lyo Vaccine | Efficacy - Other efficacy | General population |
| NCT04470609 | Safety and Immunogenicity Study of an Inactivated SARS-CoV-2 Vaccine for Preventing Against COVID-19 in People Aged ?60 Years | China | Y | Y | Vaccine | Inactivated SARS-CoV-2 vaccine | Safety | General population |
| NCT04435808 | Efficacy of Hydroxychloroquine Prophylaxis for Health Care Workers at High Risk for COVID-19 | United States | N | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04400032 | Cellular Immuno-Therapy for COVID-19 Acute Respiratory Distress Syndrome - Vanguard | Canada | N | N | Cell/Blood based | ND:MSC | Safety | Severe Cases |
| NCT04354259 | Interferon Lambda for Immediate Antiviral Therapy at Diagnosis in COVID-19 | Canada | Y | Y | Shelved drug or NME | Interferon lambda | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04352608 | Safety and Immunogenicity Study of Inactivated Vaccine for Prophylaxis of SARS CoV-2 Infection (COVID-19) | China | Y | Y | Vaccine | ND:Vaccine | Safety | General population |
| NCT04383574 | Safety and Immunogenicity Study of Inactivated Vaccine for Prevention of SARS-CoV-2 Infection(COVID-19) | China | Y | N | Vaccine | ND:Vaccine | Safety | General population |
| NCT04280588 | Fingolimod in COVID-19 | China | Y | N | Drug Repurposing | Fingolimod | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04344457 | Evaluate the Efficacy and Safety of Oral Hydroxychloroquine, Indomethacin and Zithromax in Subjects With Mild Symptoms of COVID-19 | United States | N | N | Drug Repurposing | Hydroxychloroquine, Indomethacin, Azithromycin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04351919 | Assessment of Efficacy and Safety of HCQ and Antibiotics Administrated to Patients COVID19(+) | Tunisia | N | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04336332 | Randomized Comparison of Combination Azithromycin and Hydroxychloroquine vs. Hydroxychloroquine Alone for the Treatment of Confirmed COVID-19 | United States | Y | Y | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04368988 | Evaluation of the Safety and Immunogenicity of a SARS-CoV-2 rS (COVID-19) Nanoparticle Vaccine With/Without Matrix-M Adjuvant | Australia | Y | N | Shelved drug or NME | SARS-CoV-2 rS Vaccine, Matrix-M Adjuvant | Safety | General population |
| NCT04486482 | A Clinical Study to Assess the Physiologic Effects of KB109 in Patients With COVID-19 on Gut Microbiota Structure and Function | United States | Y | N | Shelved drug or NME | KB109 | Safety | Mild/Moderate Cases |
| NCT04366271 | Clinical Trial of Allogeneic Mesenchymal Cells From Umbilical Cord Tissue in Patients With COVID-19 | Spain | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Mortality | Severe Cases |
| NCT04422678 | The Safety & Efficacy of Imatinib for the Treatment of SARS-COV-2 Induced Pneumonia | Egypt | Y | N | Drug Repurposing | Imatinib | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04333472 | Piclidenoson for Treatment of COVID-19 | Israel | Y | N | Shelved drug or NME | Piclidenoson | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04392128 | Study Evaluating the Efficacy of Hydroxychloroquine and Azithromycine in Patients With COVID-19 and Hematological Malignancies (HYACINTHE) | France | Y | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Viral load | Other |
| NCT04414124 | A Clinical Study to Assess the Natural History of COVID-19 and Effects of KB109 and Supportive Self-care in Outpatients With Mild-to-moderate COVID-19 | United States | Y | N | Shelved drug or NME | KB109 | Safety | Mild/Moderate Cases |
| NCT04389944 | Amotosalen-Ultraviolet A Pathogen-Inactivated Convalescent Plasma in Addition to Best Supportive Care and Antiviral Therapy on Clinical Deterioration in Adults Presenting With Moderate to Severe COVID-19 | Switzerland | N | N | Cell/Blood based | ND:Convalescent plasma | Safety | Mild/Moderate Cases, Severe Cases |
| NCT03891420 | A Study to Evaluate the Safety, Pharmacokinetics and Antiviral Effects of Galidesivir in Yellow Fever or COVID-19 | Brazil | Y | N | Shelved drug or NME | Galidesivir | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04397523 | Efficacy and Safety of COVID-19 Convalescent Plasma | Macedonia, the former Yugoslav Republic of | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Severe Cases |
| NCT04351152 | Phase 3 Study to Evaluate Efficacy and Safety of Lenzilumab in Patients With COVID-19 | United States | Y | N | Shelved drug or NME | Lenzilumab | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04394208 | Silymarin in COVID-19 Pneumonia | Egypt | Y | N | Drug Repurposing | Silymarin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04303299 | Various Combination of Protease Inhibitors, Oseltamivir, Favipiravir, and Hydroxychloroquine for Treatment of COVID-19 : A Randomized Control Trial | Thailand | Y | Y | Drug Repurposing, Shelved drug or NME | Oseltamivir, Hydroxychloroquine, Darunavir, Lopinavir/ritonavir, Favipiravir | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04334005 | Vitamin D on Prevention and Treatment of COVID-19 | Spain | Y | N | Drug Repurposing | Vitamin D3 | Efficacy - Mortality | Mild/Moderate Cases |
| NCT04346446 | Efficacy of Convalescent Plasma Therapy in Severely Sick COVID-19 Patients | India | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Severe Cases |
| NCT04357457 | Efficacy of Intravenous Almitrine in Reducing the Need for Mechanical Ventilation in Patients With Hypoxemic Acute Respiratory Failure Due to Covid-19-related Pneumonia | France | Y | N | Drug Repurposing | Almitrine | Efficacy - Other efficacy | Severe Cases |
| NCT04456049 | Randomized Open Label Phase II Study to Evaluate the Efficacy of Enzalutamide in High Risk Male Outpatients With COVID-19 | Switzerland | Y | N | Drug Repurposing | Enzalutamide | Efficacy - Clinical improvement (except WHO scale) | Other |
| NCT04405271 | TAF/FTC for Pre-exposure Prophylaxis of COVID-19 in Healthcare Workers (CoviPrep Study) | Argentina | Y | N | Drug Repurposing | Emtricitabine/Tenofovir Alafenamide | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04348877 | Plasma Rich Antibodies From Recovered Patients From COVID19 | Egypt | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Viral load | Severe Cases |
| NCT04344951 | Chloroquine Phosphate Against Infection by the Novel Coronavirus SARS-CoV-2 (COVID-19): The HOPE Open-Label, Non Randomized Clinical Trial | Greece | N | N | Drug Repurposing | Chloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04487574 | A Study to Assess the Efficacy and Safety of XC221 in Patients With COVID-19 | Russian Federation | Y | N | Shelved drug or NME | XC221 | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04349241 | Efficacy and Safety of Favipiravir in Management of COVID-19 | Egypt | Y | N | Drug Repurposing | Favipiravir | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04360876 | Targeted Steroids for ARDS Due to COVID-19 Pneumonia: A Pilot Randomized Clinical Trial | United States | Y | N | Drug Repurposing | Dexamethasone | Efficacy - Other efficacy | Severe Cases |
| NCT04452812 | Statistical and Epidemiological Study Based on the Use of Convalescent Plasma for the Management of Patients With COVID-19 | Mexico | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04362176 | Passive Immunity Trial of Nashville II for COVID-19 | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04331600 | Chloroquine as Antiviral Treatment in Coronavirus Infection 2020 | Poland | Y | N | Drug Repurposing | Chloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04409925 | DISmantling COvid iNduced Neutrophil ExtraCellular Traps (DISCONNECT-1) | Canada | N | N | Drug Repurposing | Dornase alfa | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04342897 | A Study of LY3127804 in Participants With COVID-19 | United States | Y | N | Shelved drug or NME | LY3127804 | Efficacy - Oxygen parameters | Severe Cases |
| NCT04487886 | Duvelisib Ameliorates Manifestations of Pneumonia in Established Novel Coronavirus Infection (COVID-19) | United States | Y | N | Drug Repurposing | Duvelisib | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04377308 | Fluoxetine to Reduce Intubation and Death After COVID19 Infection | United States | N | N | Drug Repurposing | Fluoxetine | Efficacy - Infection rate (prevention) | Mild/Moderate Cases, Severe Cases |
| NCT04360122 | Levamisole and Isoprinosine in Immune-prophylaxis of Egyptian Healthcare Workers Facing COVID-19 | Egypt | Y | N | Drug Repurposing | Levamisole, Isoprinosine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04354428 | Treatment for COVID-19 in High-Risk Adult Outpatients | United States | Y | Y | Drug Repurposing | Hydroxychloroquine, Ascorbic acid, Folic acid, Azithromycin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04350736 | First in Human SAD and MAD Study of Inhaled TD-0903, a Potential Treatment for ALI Associated With COVID-19 | United Kingdom | Y | N | Shelved drug or NME | TD-0903 | Safety | General population |
| NCT04347681 | Potential Efficacy of Convalescent Plasma to Treat Severe COVID-19 and Patients at High Risk of Developing Severe COVID-19 | Saudi Arabia | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04348513 | Triiodothyronine for the Treatment of Critically Ill Patients With COVID-19 Infection | Greece | Y | N | Drug Repurposing | Triiodothyronine | Efficacy - Other efficacy | Severe Cases |
| NCT04341116 | Study of TJ003234 (Anti-GM-CSF Monoclonal Antibody) in Subjects With Severe Coronavirus Disease 2019 (COVID-19) | United States | Y | Y | Shelved drug or NME | TJ003234 (Anti-GM-CSF Monoclonal Antibody) | Efficacy - WHO scale | Mild/Moderate Cases |
| NCT04377568 | Efficacy of Human Coronavirus-immune Convalescent Plasma for the Treatment of COVID-19 Disease in Hospitalized Children | Canada | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Other |
| NCT04373460 | Convalescent Plasma to Limit SARS-CoV-2 Associated Complications | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Mild/Moderate Cases |
| NCT04350931 | Application of BCG Vaccine for Immune-prophylaxis Among Egyptian Healthcare Workers During the Pandemic of COVID-19 | Egypt | Y | N | Drug Repurposing | BCG Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04458363 | Convalescent Plasma in Pediatric COVID-19 | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Safety | Other |
| NCT04373044 | Baricitinib, Placebo and Antiviral Therapy for the Treatment of Patients With Moderate and Severe COVID-19 | United States | N | N | Drug Repurposing, Shelved drug or NME | Baricitinib, Hydroxychloroquine | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04389840 | Dociparstat for the Treatment of Severe COVID-19 in Adults at High Risk of Respiratory Failure | United States | Y | N | Shelved drug or NME | Dociparastat | Efficacy - Other efficacy | Severe Cases |
| NCT04447469 | Study of Mavrilimumab (KPL-301) in Participants Hospitalized With Severe Corona Virus Disease 2019 (COVID-19) Pneumonia and Hyper-inflammation | United States | Y | N | Shelved drug or NME | Mavrilimumab | Efficacy - Oxygen parameters | Severe Cases |
| NCT04341389 | A Phase II Clinical Trial to Evaluate the Recombinant Vaccine for COVID-19 (Adenovirus Vector) | China | Y | Y | Vaccine | ND:Vaccine | Safety | General population |
| NCT04359654 | Nebulised Dornase Alfa for Treatment of COVID-19 | United Kingdom | Y | N | Drug Repurposing | Dornase alfa | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04468971 | REgulatory T Cell infuSion fOr Lung Injury Due to COVID-19 PnEumonia | United States | Y | Y | Shelved drug or NME | CK0802 | Safety | Severe Cases |
| NCT04491994 | Clearing the Fog: Is Hydroxychloroquine Effective in Reducing COVID-19 Progression | Pakistan | Y | N | Drug Repurposing | Hydoxychloroquine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04413864 | Immunomodulatory Profile of Dexmedetomidine Sedation in Patients Recovering After ARDS Covid-19 | France | N | N | Drug Repurposing | Dexmedetomidine | Efficacy - Other efficacy | Severe Cases |
| NCT04374526 | Early transfusIon of Convalescent Plasma in Elderly COVID-19 Patients. to Prevent Disease Progression. | Italy | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04375098 | Efficacy and Safety of Early COVID-19 Convalescent Plasma in Patients Admitted for COVID-19 Infection | Chile | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04317040 | CD24Fc as a Non-antiviral Immunomodulator in COVID-19 Treatment | United States | Y | N | Shelved drug or NME | CD24Fc | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04409509 | Treatment With CSL312 in Adults With Coronavirus Disease 2019 (COVID-19) | United States | Y | N | Shelved drug or NME | Garadacimab | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04326790 | The GReek Study in the Effects of Colchicine in Covid-19 cOmplications Prevention | Greece | Y | N | Drug Repurposing | Colchicine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04339816 | Azithromycin Added to Hydrochloroquine in Patients Admitted to Intensive Care With COVID-19: Randomised Controlled Trial | Czech Republic | Y | N | Drug Repurposing | Azithromycin, Hydroxychloroquine | Efficacy - Other efficacy | Severe Cases |
| NCT04453488 | Clinical Trial to Evaluate the Efficacy of RUTI® Against SARS-COV-2 Infection (COVID-19) in Healthcare Workers | Spain | Y | N | Vaccine | ND:RUTI Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04349371 | Saved From COVID-19 | United States | Y | N | Drug Repurposing | Chloroquine | Efficacy - Other efficacy | Exposed individuals (family members, HCP) |
| NCT04364893 | Angiotensin Receptor Blockers and Angiotensin-converting Enzyme Inhibitors and Adverse Outcomes in Patients With COVID19 | Brazil | Y | N | Drug Repurposing | Angiotensin Receptor Blockers, Angiotensin-converting Enzyme Inhibitors | Efficacy - Other efficacy | Severe Cases |
| NCT04351516 | Test and Treat COVID 65plus+ | Germany | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04451291 | Study of Decidual Stromal Cells to Treat COVID-19 Respiratory Failure | Canada | N | N | Cell/Blood based | ND:Decidual Stromal Cells | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04345653 | Hydroxychloroquine as Chemoprevention for COVID-19 for High Risk Healthcare Workers | United States | N | N | Drug Repurposing | Hydroxychloroquine | Other | Exposed individuals (family members, HCP) |
| NCT04468646 | To Determine the Efficacy of Neurokinin 1 Receptor Antagonist as a Therapeutic Tool Against Cytokine Storm and Respiratory Failure in Covid-19 Patients | Pakistan | Y | N | Drug Repurposing | Aprepitant | Efficacy - WHO scale | Severe Cases |
| NCT04331054 | Protective Role of Inhaled Steroids for Covid-19 Infection | France | Y | N | Drug Repurposing | Budesonide/formoterol | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04358406 | Rhu-pGSN for Severe Covid-19 Pneumonia | United States | Y | N | Shelved drug or NME | Recombinant human plasma gelsolin | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04346368 | Bone Marrow-Derived Mesenchymal Stem Cell Treatment for Severe Patients With Coronavirus Disease 2019 (COVID-19) | China | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Oxygen parameters | Severe Cases |
| NCT04392973 | FAvipiravir and HydroxyChloroquine Combination Therapy | Saudi Arabia | Y | N | Drug Repurposing | Favipiravir, Hydroxychloroquine | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04396067 | Combination With Inhibitor of Neutrophil Elastase (All-trans Retinoic Acid ) and Isotretinoin May Enhances Neutralizing Antibodies in COVID -19 Infected Patients Better Than COVID-19 Inactivated Vaccines | Egypt | Y | N | Drug Repurposing | Alvelestat, Isotretinoin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04335071 | Tocilizumab in the Treatment of Coronavirus Induced Disease (COVID-19) | Switzerland | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04395170 | Convalescent Plasma (PC) and Human Intravenous Anti-COVID-19 Immunoglobulin (IV Anti COVID-19 IgG) in Patients Hospitalized for COVID-19. | Colombia | Y | Y | Cell/Blood based | ND:Convalescent plasma, ND:Human Immunoglobulin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04459676 | Study to Assess Efficacy and Safety Relative to Standard of Care in Patients With COVID-19 Pneumonia | Brazil | Y | N | Shelved drug or NME | ANG-3777 | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04345276 | Efficacy and Safety of Ganovo (Danoprevir) Combined With Ritonavir in the Treatment of SARS-CoV-2 Infection | China | N | N | Drug Repurposing, Shelved drug or NME | Danoprevir, Ritonavir | Efficacy - Oxygen parameters | Mild/Moderate Cases |
| NCT04291729 | Evaluation of Ganovo (Danoprevir ) Combined With Ritonavir in the Treatment of SARS-CoV-2 Infection | China | N | N | Drug Repurposing, Shelved drug or NME | Danoprevir, Ritonavir, Interferon(NOS) | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04338906 | Combination Therapy With Camostat Mesilate + Hydroxychloroquine for COVID-19 | Germany | Y | N | Drug Repurposing | Camostat | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04386447 | Phase II RCT to Assess Efficacy of Intravenous Administration of Oxytocin in Patients Affected by COVID-19 | Italy | Y | Y | Drug Repurposing | Oxytocin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04286503 | The Clinical Study of Carrimycin on Treatment Patients With COVID-19 | China | Y | N | Shelved drug or NME | Carrimycin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04380376 | Low-doses Melphalan Inhalation in Patients With COVID-19 (CoronavIrus Disease 2019) Pneumonia | Russian Federation | N | N | Drug Repurposing | Melphalan | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04288102 | Treatment With Human Umbilical Cord-derived Mesenchymal Stem Cells for Severe Corona Virus Disease 2019 (COVID-19) | China | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04352803 | Adipose Mesenchymal Cells for Abatement of SARS-CoV-2 Respiratory Compromise in COVID-19 Disease | United States | N | N | Cell/Blood based | ND:MSCs | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04350580 | Polyvalent Immunoglobulin in COVID-19 Related ARds | France | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04487990 | CoV-Hep Study: Regional Anticoagulation Modalities in Continuous Venous Venous Hemodialysis in Patients With COVID-19 | Brazil | Y | N | Drug Repurposing | Heparin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04466241 | Combination Therapies to Reduce Carriage of SARS-Cov-2 and Improve Outcome of COVID-19 in Ivory Coast: a Phase Randomized IIb Trial | Ivory Coast | Y | Y | Drug Repurposing | Telmisartan, Atorvastatin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04428073 | Therapeutic Vaccine Trial of COVID-19 for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection | United States | Y | Y | Vaccine | ND:Vaccine | Safety | Mild/Moderate Cases |
| NCT04365582 | OUTpatient Treatment of COVID-19 in Patients With Risk Factor for Poor Outcome | France | Y | N | Drug Repurposing | Azithromycin, Hydroxychloroquine, Lopinavir/ritonavir | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04353674 | Modulation of Hyperinflammation in COVID-19 | United States | Y | N | Cell/Blood based | ND:Neutrophil modulation | Efficacy - Other efficacy | Severe Cases |
| NCT04473261 | Efficacy of Iodine Complex Against COVID-19 Patients | Pakistan | Y | Y | Shelved drug or NME | Iodine complex | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04348305 | Hydrocortisone for COVID-19 and Severe Hypoxia | Denmark | Y | N | Drug Repurposing | Hydrocortisone | Efficacy - Other efficacy | Severe Cases |
| NCT04407286 | Vitamin D Testing and Treatment for COVID 19 | United States | N | N | Drug Repurposing | Vitamin D3 | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04401410 | Anti-SARS Cov-2 T Cell Infusions for COVID 19 | United States | N | N | Cell/Blood based | ND:T-cells | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04344600 | Peginterferon Lambda-1a for the Prevention and Treatment of SARS-CoV-2 (COVID-19) Infection | United States | Y | N | Shelved drug or NME | Peg-Interferon lambda-1a | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04377503 | Tocilizumab Versus Methylprednisolone in the Cytokine Release Syndrome of Patients With COVID-19 | Brazil | Y | N | Drug Repurposing | Tocilizumab | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04395456 | A Phase 2 Study of the C3 Inhibitor AMY-101 in Patients With ARDS Due to COVID-19 (SAVE) | Greece | Y | N | Shelved drug or NME | AMY-101 | Efficacy - Mortality | Severe Cases |
| NCT04335305 | Checkpoint Blockade in COVID-19 Pandemic | Spain | Y | N | Drug Repurposing | Pembrolizumab, Tocilizumab | Efficacy - Oxygen parameters | Severe Cases |
| NCT04372589 | Antithrombotic Therapy to Ameliorate Complications of COVID-19 ( ATTACC ) | Canada | Y | N | Drug Repurposing | Heparin | Efficacy - Other efficacy | Severe Cases |
| NCT04273321 | Efficacy and Safety of Corticosteroids in COVID-19 | China | Y | N | Drug Repurposing | Methylprednisolone | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04474340 | COVID-19 Convalescent Plasma Treatment in SARS-CoV-2 Infected Patients | Kuwait | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04403477 | Convalescent Plasma Therapy in Severe COVID-19 Infection | Bangladesh | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| NCT04351490 | Impact of Zinc and Vitamin D3 Supplementation on the Survival of Aged Patients Infected With COVID-19 | France | Y | N | Drug Repurposing | Zinc, Vitamin D3 | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04375735 | London's Exogenous Surfactant Study for COVID19 | United States | Y | N | Drug Repurposing | Bovine Lipid Extract Surfactant | Safety | Mild/Moderate Cases |
| NCT04353336 | Efficacay of Chloroquine or Hydroxychloroquine in COVID-19 Treatment | Egypt | Y | N | Drug Repurposing | Chloroquine, Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04397796 | Study of the Safety of Therapeutic Tx With Immunomodulatory MSC in Adults With COVID-19 Infection Requiring Mechanical Ventilation | United States | Y | N | Cell/Blood based | ND:Stem cells | Safety | Severe Cases |
| NCT04399889 | hCT-MSCs for COVID19 ARDS | United States | Y | N | Cell/Blood based | ND:MSC | Safety | Severe Cases |
| NCT04331665 | Study of the Efficacy and Safety of Ruxolitinib to Treat COVID-19 Pneumonia | Canada | N | N | Drug Repurposing | Ruxolitinib | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04362111 | Early Identification and Treatment of Cytokine Storm Syndrome in Covid-19 | United States | Y | N | Drug Repurposing | Anakinra | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04378920 | A Study of Trans Crocetin in Patients With Acute Respiratory Distress Syndrome Due to COVID-19 Disease (LEAF-4L7520/4L6715) | France | N | N | Shelved drug or NME | LEAF-4L7520, LEAF-4L6715 | Efficacy - Oxygen parameters | Severe Cases |
| NCT04318444 | Hydroxychloroquine Post Exposure Prophylaxis for Coronavirus Disease (COVID-19) | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04389671 | The Safety and Preliminary Efficacy of Lucinactant in Adults With COVID-19 | United States | N | N | Drug Repurposing | Lucinactant | Efficacy - Oxygen parameters | Severe Cases |
| NCT04395105 | Dexamethasone for COVID-19 Related ARDS: a Multicenter, Randomized Clinical Trial | Argentina | Y | N | Drug Repurposing | Dexamethasone | Efficacy - Other efficacy | Severe Cases |
| NCT04402203 | Study on Safety and Efficacy of Favipiravir (Favipira) for COVID-19 Patient in Selected Hospitals of Bangladesh | Bangladesh | Y | N | Drug Repurposing | Favipiravir | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04438837 | Hydroxychloroquine Post-Exposure Prophylaxis for Coronavirus Disease (COVID-19) Among Health-Care Workers | Israel | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04467840 | Opaganib, a Sphingosine Kinase-2 (SK2) Inhibitor in COVID-19 Pneumonia | United States | Y | N | Shelved drug or NME | Opaganib | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04402944 | Pulmozyme to Improve COVID-19 ARDS Outcomes | United States | Y | N | Drug Repurposing | Dornase alfa | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04407390 | Effects of Nicotinamide Riboside on the Clinical Outcome of Covid-19 in the Elderly | Denmark | Y | N | Shelved drug or NME | Nicotinamide Riboside | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04318015 | Hydroxychloroquine Chemoprophylaxis in Healthcare Personnel in Contact With COVID-19 Patients (PHYDRA Trial) | Mexico | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04341038 | Clinical Trial to Evaluate Methylprednisolone Pulses and Tacrolimus in Patients With COVID-19 Lung Injury | Spain | Y | N | Drug Repurposing | Methylprednisolone, Tacrolimus | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04381988 | A Study of Hydroxychloroquine vs Placebo to Prevent COVID-19 Infection in Patients Receiving Radiotherapy | United States | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Other |
| NCT04456361 | Use of Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome Caused by COVID-19 | Mexico | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04330144 | Hydroxychloroquine as Post Exposure Prophylaxis for SARS-CoV-2(HOPE Trial) | Korea, Republic of | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | Mild/Moderate Cases, Severe Cases |
| NCT04392713 | Efficacy of Ivermectin in COVID-19 | Pakistan | Y | N | Drug Repurposing | Ivermectin | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04407689 | InterLeukin-7 to Improve Clinical Outcomes in Lymphopenic pAtients With COVID-19 Infection FR BL Cohort | France | Y | N | Shelved drug or NME | CYT107 | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04379076 | InterLeukin-7 (CYT107) to Improve Clinical Outcomes in Lymphopenic pAtients With COVID-19 Infection UK Cohort | United Kingdom | Y | N | Shelved drug or NME | Interleukin-7 (CYT107) | Other | Mild/Moderate Cases |
| NCT04445311 | Ivermectin in Treatment of COVID-19 | Egypt | Y | N | Drug Repurposing | Ivermectin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04377997 | Safety and Efficacy of Therapeutic Anticoagulation on Clinical Outcomes in Hospitalized Patients With COVID-19 | United States | Y | N | Drug Repurposing | Enoxaparin | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04345848 | Preventing COVID-19 Complications With Low- and High-dose Anticoagulation | Switzerland | Y | N | Drug Repurposing | Enoxaparin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04335552 | Pragmatic Factorial Trial of Hydroxychloroquine, Azithromycin, or Both for Treatment of Severe SARS-CoV-2 Infection | United States | Y | Y | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - WHO scale | Severe Cases |
| NCT04436458 | Niclosamide In Moderate COVID-19 | United States | Y | N | Drug Repurposing | Niclosamide | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04343963 | Pyridostigmine in Severe SARS-CoV-2 Infection | Mexico | Y | N | Drug Repurposing | Pyridostigmine | Efficacy - Mortality | Severe Cases |
| NCT04374149 | Therapeutic Plasma Exchange Alone or in Combination With Ruxolitinib in COVID-19 Associated CRS | United States | Y | N | Drug Repurposing, Other | Ruxolitinib, ND:Procedure | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04419610 | RAS and Coagulopathy in COVID19 | United Kingdom | Y | N | Shelved drug or NME | TRV027 | Efficacy - Other efficacy | Other |
| NCT04401475 | A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of EB05 + SOC vs. Placebo + SOC in Adult Hospitalized Patients With Moderate to Severe COVID-19 | United States | Y | N | Shelved drug or NME | EB05 | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04390061 | TOFAcitinib Plus Hydroxycloroquine vs Hydroxycloroquine in Patients With COVID-19 Interstitial Pneumonia | Italy | Y | N | Drug Repurposing | Tofacitinib | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04359329 | Estrogen Patch for COVID-19 Symptoms | United States | Y | N | Drug Repurposing | Estrogen patch | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04422561 | Prophylactic Ivermectin in COVID-19 Contacts | Egypt | N | N | Drug Repurposing | Ivermectin | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04403555 | Ivermectin and Doxycycine in COVID-19 Treatment | Egypt | Y | N | Drug Repurposing | Doxycycline, Ivermectin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04351295 | Efficacy of Faviprevir in COVID-19 Treatment | Egypt | Y | N | Drug Repurposing | Favipiravir | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04401293 | Full Dose Heparin Vs. Prophylactic Or Intermediate Dose Heparin in High Risk COVID-19 Patients | United States | Y | N | Drug Repurposing | Enoxaparin | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04387240 | Evaluating the Efficacy of Artesunate in Adults With Mild Symptoms of COVID-19 | Saudi Arabia | Y | N | Drug Repurposing | Artesunate | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04426201 | InterLeukin-7 to Improve Clinical Outcomes in Lymphopenic pAtients With COVID-19 Infection ( ILIAD-7-US-O ) | United States | Y | N | Shelved drug or NME | CYT107 | Efficacy - Other efficacy | Other |
| NCT04345406 | Angiotensin Converting Enzyme Inhibitors in Treatment of Covid 19 | Egypt | Y | N | Drug Repurposing | Captopril, Enalapril | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04388709 | Interferon Lambda Therapy for COVID-19 | United States | Y | N | Drug Repurposing | Peginterferon lambda-1a | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04433988 | Efficacy of Pentoxifylline as Add on Therapy in COVID19 Patients | Egypt | Y | N | Drug Repurposing | Pentoxifylline | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04374019 | Novel Agents for Treatment of High-risk COVID-19 Positive Patients | United States | Y | Y | Drug Repurposing | Hydroxychloroquine, Azithromycin, Ivermectin, Camostat, Artemisinin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04460443 | Sofosbuvir With and Without Ribavirin in Treatment of COVID 19 | Egypt | Y | Y | Drug Repurposing | Sofosbuvir, Ribavirin, Daclatasvir | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04363853 | Tocilizumab Treatment in Patients With COVID-19 | Mexico | N | N | Drug Repurposing | Tocilizumab | Efficacy - Other efficacy | Severe Cases |
| NCT04361032 | Assessment of Efficacy and Safety of Tocilizumab Compared to DefeROxamine, Associated With Standards Treatments in COVID-19 (+) Patients Hospitalized In Intensive Care in Tunisia | Tunisia | Y | N | Drug Repurposing | Tocilizumab, Deferoxamine | Efficacy - Mortality | Severe Cases |
| NCT04424901 | Trial of Open Label Dipyridamole- In Hospitalized Patients With COVID-19 | United States | Y | N | Drug Repurposing | Dipyridamole | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04374552 | Asymptomatic COVID-19 Trial | United States | Y | N | Drug Repurposing | Hydroxychloroquine, Azithromycin | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04408040 | Use of Convalescent Plasma for COVID-19 | United States | N | Y | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04359901 | Sarilumab for Patients With Moderate COVID-19 Disease | United States | Y | N | Drug Repurposing | Sarilumab | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04371107 | Proactive Care of Ambulatory COVID19 Patients | France | Y | N | Drug Repurposing | Azithromycin | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04459247 | Short Term, High Dose Vitamin D Supplementation for COVID-19 | India | Y | N | Drug Repurposing | Vitamin D3 | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04425915 | Efficacy of Convalescent Plasma Therapy in Patients With COVID-19 | India | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04351347 | The Efficacy of Ivermectin and Nitazoxanide in COVID-19 Treatment | Egypt | Y | N | Drug Repurposing | Ivermectin, Nitazoxanide, Chloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04313127 | Phase I Clinical Trial of a COVID-19 Vaccine in 18-60 Healthy Adults | China | N | N | Vaccine | ND:Vaccine | Safety | General population |
| NCT04447534 | Zinc With Chloroquine/Hydroxychloroquine in Treatment of COVID-19 | Egypt | N | N | Drug Repurposing | Zinc | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04469491 | Treatment of COVID-19 by Nebulization of Inteferon Beta 1b Efficiency and Safety Study | France | Y | N | Drug Repurposing | Interferon-1-beta | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04399746 | Ivermectin-Azithromycin-Cholecalciferol (IvAzCol) Combination Therapy for COVID-19 | Mexico | N | N | Drug Repurposing | Ivermectin, Azithromycin, Cholecalciferol | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04445467 | An Adaptive Randomised Placebo Controlled Phase II Trial of Antivirals for COVID-19 Infection | United States | Y | N | Drug Repurposing | Favipiravir | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04393051 | Baricitinib Compared to Standard Therapy in Patients With COVID-19 | Italy | Y | N | Drug Repurposing | Baricitinib | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04391101 | Convalescent Plasma for the Treatment of Severe SARS-CoV-2 (COVID-19) | Colombia | Y | N | Cell/Blood based | ND:Convalescent plasma | Safety | Severe Cases |
| NCT04479163 | Prevention of Severe Covid-19 in Infected Elderly by Early Administration of Convalescent Plasma With High-titers of Antibody Against SARS-CoV2 | Cuba | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Oxygen parameters | Other |
| NCT04472611 | Colchicine/Statins for the Prevention of COVID-19 Complications (COLSTAT) Trial | United States | Y | N | Drug Repurposing | Colchicine, Rosuvastatin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04343261 | Convalescent Plasma in the Treatment of COVID 19 | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| NCT04356937 | Efficacy of Tocilizumab on Patients With COVID-19 | United States | Y | N | Drug Repurposing | Tocilizumab | Efficacy - Other efficacy | Severe Cases |
| NCT04475302 | BCG Vaccine in Reducing Morbidity and Mortality in Elderly Individuals in COVID-19 Hotspots | India | Y | N | Drug Repurposing | BCG Vaccine | Efficacy - Mortality | Other |
| NCT04344535 | Convalescent Plasma vs. Standard Plasma for COVID-19 | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Oxygen parameters | Severe Cases |
| NCT04371367 | Avdoralimab an Anti-C5aR Antibody, in Patients With COVID-19 Severe Pneumonia ( FORCE ) | France | Y | N | Shelved drug or NME | Avdoralimab | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04359862 | Sedation With Sevoflurane Versus Propofol in Patients With Acute Respiratory Distress Syndrome Caused by COVID19 Infection | Spain | Y | N | Drug Repurposing | Sevoflurane | Efficacy - Oxygen parameters | Severe Cases |
| NCT04372082 | Hydroxychloroquine or Diltiazem-Niclosamide for the Treatment of COVID-19 | France | Y | Y | Drug Repurposing | Diltiazem, Niclosamide, Hydroxychloroquine | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04442048 | Immunization With IMM-101 vs Observation for Prevention of Respiratory and Severe COVID-19 Related Infections in Cancer Patients at Increased Risk of Exposure | Canada | Y | N | Shelved drug or NME | IMM-101 | Efficacy - Infection rate (prevention) | Other |
| NCT04363502 | Use of the Interleukin-6 Inhibitor Clazakizumab in Patients With Life-threatening COVID-19 Infection | United States | Y | N | Shelved drug or NME | Clazakizumab | Efficacy - Other efficacy | Severe Cases |
| NCT04392427 | New Antiviral Drugs for Treatment of COVID-19 | Egypt | Y | N | Drug Repurposing | Nitazoxanide, Ribavirin, Ivermectin | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04404361 | PRE-VENT Study in Hospitalized Patients With Severe COVID-19 With or Without Cancer | United States | Y | N | Shelved drug or NME | Pacritinib | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04382924 | Safety and Efficacy of NP-120 (Ifenprodil) for the Treatment of Hospitalized Patient With Confirmed COVID-19 Disease | Australia | Y | N | Shelved drug or NME | Ifenprodil | Efficacy - WHO scale | Severe Cases |
| NCT04398290 | iNOPulse for COVID-19 | United States | Y | N | Drug Repurposing | Nitric oxide | Safety | Mild/Moderate Cases |
| NCT04445623 | Prasugrel in Severe COVID-19 Pneumonia | Italy | Y | N | Drug Repurposing | Prasugrel | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04344548 | Phase I / II Clinical Study of Immunotherapy Based on Adoptive Cell Transfer as a Therapeutic Alternative for Patients With COVID-19 in Colombia | Colombia | N | N | Cell/Blood based | ND:NK-cells | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04408183 | GLS-1200Topical Nasal Spray to Prevent SARS-CoV-2 Infection (COVID-19) in Health Care Personnel | United States | Y | N | Shelved drug or NME | GLS-1200 | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04482673 | Vitamin D Supplementation in the Prevention and Mitigation of COVID-19 Infection | United States | Y | N | Drug Repurposing | Vitamin D3 | Other | Other |
| NCT04334460 | Safety and Antiviral Activity of BLD-2660 in COVID-19 Hospitalized Subjects | United States | Y | N | Shelved drug or NME | BLD-2660 | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04328012 | COVID MED Trial - Comparison Of Therapeutics for Hospitalized Patients Infected With SARS-CoV-2 | United States | Y | Y | Drug Repurposing | Lopinavir/ritonavir, Hydroxychloroquine, Losartan | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04445389 | Safety and Immunogenicity Study of GX-19, a COVID-19 Preventive DNA Vaccine in Healthy Adults | Korea, Republic of | Y | N | Vaccine | GX-19 | Safety | Other |
| NCT04468009 | Treatment of Critically Ill Patients With Covid-19 With Convalescent Plasma | Argentina | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Mortality | Severe Cases |
| NCT04261517 | Efficacy and Safety of Hydroxychloroquine for Treatment of COVID-19 | China | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04405570 | A Safety, Tolerability and Efficacy of EIDD-2801 to Eliminate Infectious Virus Detection in Persons With COVID-19 | United States | Y | N | Shelved drug or NME | EIDD-2801 | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04382950 | Combination of Recombinant Bacterial ACE2 Receptors -Like Enzyme of B38-CAP and Isotretinoin Could be Promising COVID-19 Infection- and Lung Injury Preventing Drug Better Than Recombinant Human ACE2 | Israel | Y | N | Drug Repurposing, Shelved drug or NME | rbACE2, Isotretinoin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04450004 | Safety, Tolerability and Immunogenicinity of a Coronavirus-Like Particle COVID-19 Vaccine in Adults Aged 18-55 Years. | United States | Y | N | Vaccine | ND:Vaccine | Safety | Other |
| NCT04366245 | Clinical Trial to Evaluate the Efficacy of Treatment With Hyperimmune Plasma Obtained From Convalescent Antibodies of COVID-19 Infection | Spain | Y | N | Cell/Blood based | ND:Convalescent plasma | Safety | Mild/Moderate Cases |
| NCT04367831 | Intermediate or Prophylactic-Dose Anticoagulation for Venous or Arterial Thromboembolism in Severe COVID-19 | United States | Y | N | Drug Repurposing | Heparin, Enoxaparin/Lovenox | Efficacy - Other efficacy | Severe Cases |
| NCT04373824 | Max Ivermectin- COVID 19 Study Versus Standard of Care Treatment for COVID 19 Cases. A Pilot Study | India | Y | N | Drug Repurposing | Ivermectin | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04319731 | A Pilot Study of Human Amniotic Fluid for COVID19 Associated Respiratory Failure | United States | N | N | Cell/Blood based | ND:Amniotic fluid | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04493242 | Extracellular Vesicle Infusion Therapy for Severe COVID-19 | United States | Y | N | Shelved drug or NME | DB-001 | Efficacy - Mortality | Severe Cases |
| NCT04385199 | Convalescent Plasma for Patients With COVID-19 | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04366323 | Clinical Trial to Assess the Safety and Efficacy of Intravenous Administration of Allogeneic Adult Mesenchymal Stem Cells of Expanded Adipose Tissue in Patients With Severe Pneumonia Due to COVID-19 | Spain | Y | N | Cell/Blood based | ND:Stem cells | Safety | Severe Cases |
| NCT04257656 | A Trial of Remdesivir in Adults With Severe COVID-19 | China | Y | N | Shelved drug or NME | Remdesivir | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04492514 | Mavrilimumab to Reduce Progression of Acute Respiratory Failure in COVID-19 Pneumonia and Systemic Hyper-inflation | United States | Y | N | Shelved drug or NME | Mavrilimumab | Efficacy - Mortality | Severe Cases |
| NCT04477993 | Ruxolitinib for Acute Respiratory Disorder Syndrome Due to COVID-19 | Brazil | Y | N | Drug Repurposing | Ruxolitinib | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04338698 | Hydroxychloroquine, Oseltamivir and Azithromycin for the Treatment of COVID-19 Infection: An RCT | Pakistan | Y | Y | Drug Repurposing | Hydroxychloroquine, Oseltamivir, Azithromycin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04375397 | Study of Oral Ibrutinib Capsules to Assess Respiratory Failure in Adult Participants With Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) and Pulmonary Injury | United States | Y | N | Drug Repurposing | Ibrutinib | Efficacy - Other efficacy | Severe Cases |
| NCT04439045 | Efficacy and Safety of VPM1002 in Reducing SARS-CoV-2 (COVID-19) Infection Rate and Severity | United States | Y | N | Vaccine | ND:VPM1002 vaccine | Efficacy - Infection rate (prevention) | Other |
| NCT04463004 | Mavrilimumab to Reduce Progression of Acute Respiratory Failure in COVID-19 Pneumonia and Systemic Hyper-inflammation | United States | Y | N | Shelved drug or NME | Mavrilimumab | Efficacy - Mortality | Severe Cases |
| NCT04399980 | Mavrilimumab to Reduce Progression of Acute Respiratory Failure in COVID-19 Pneumonia and Systemic Hyper-inflammation | United States | Y | N | Shelved drug or NME | Mavrilimumab | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04397510 | Nebulized Heparin for the Treatment of COVID-19 Induced Lung Injury | United States | Y | N | Drug Repurposing | Heparin | Efficacy - Oxygen parameters | Severe Cases |
| NCT04457609 | Administration of Allogenic UC-MSCs as Adjuvant Therapy for Critically-Ill COVID-19 Patients | Indonesia | Y | N | Drug Repurposing, Cell/Blood based | Oseltamivir, Azithromycin, ND:Stem cells | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04470427 | A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19 | United States | Y | N | Vaccine | mRNA-1273 vaccine | Efficacy - Infection rate (prevention) | General population |
| NCT04364737 | CONTAIN COVID-19: Convalescent Plasma to Limit COVID-19 Complications in Hospitalized Patients | United States | Y | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - WHO scale | Severe Cases |
| NCT04405076 | Dose-Confirmation Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1273 COVID-19 Vaccine in Adults Aged 18 Years and Older | United States | Y | N | Vaccine | ND:Vaccine | Safety | General population |
| NCT04349631 | A Clinical Trial to Determine the Safety and Efficacy of Hope Biosciences Autologous Mesenchymal Stem Cell Therapy (HB-adMSCs) to Provide Protection Against COVID-19 | United States | N | N | Cell/Blood based | ND:Stem cells | Efficacy - Other efficacy | Exposed individuals (family members, HCP) |
| NCT04379271 | A Study to Evaluate the Efficacy, Safety and Tolerability of IMU-838 as Addition to Investigator's Choice of Standard of Care Therapy, in Patients With Coronavirus Disease 19 (COVID-19) | United States | Y | N | Shelved drug or NME | IMU-838 | Efficacy - Other efficacy | Mild/Moderate Cases |
| NCT04414241 | Hydroxychloroquine to Prevent SARS-CoV-2 Infection | Peru | Y | N | Drug Repurposing | Hydroxychloroquine | Efficacy - Infection rate (prevention) | General population |
| NCT04345601 | Mesenchymal Stromal Cells for the Treatment of SARS-CoV-2 Induced Acute Respiratory Failure (COVID-19 Disease) | United States | N | N | Cell/Blood based | ND:MSC | Safety | Severe Cases |
| NCT04399356 | Niclosamide for Mild to Moderate COVID-19 | United States | Y | N | Drug Repurposing | Niclosamide | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04351191 | PRophylaxis of Exposed COVID-19 Individuals With Mild Symptoms Using choloroquinE Compounds | Pakistan | Y | Y | Drug Repurposing | Hydroxychloroquine, Chloroquine | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04377334 | Mesenchymal Stem Cells (MSCs) in Inflammation-Resolution Programs of Coronavirus Disease 2019 (COVID-19) Induced Acute Respiratory Distress Syndrome (ARDS) | Germany | Y | N | Cell/Blood based | ND:Stem cells | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| ACTRN12620 000557932 |
Therapies to prevent progression of COVID-19, including Hydroxychloroquine, Azithromycin, Zinc, Vitamin D, Vitamin B12 with or without Vitamin C, a multi-centre, international, randomized trial: The International ALLIANCE Study | Australia | Y | Y | Drug Repurposing | Hydroxychloroquine, Azithromycin, Zinc Citrate, Vitamin D3, Vitamin B12, Ascorbic acid | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| ACTRN12620 000788976 |
Safety and efficacy of intranasal delivery of BromAc® (Bromelain & Acetylcysteine) in swab positive SARS-CoV-2 patients – inactivating the COVID-19 virus by cleavage of the spike and other glycoproteins | Australia | N | N | Drug Repurposing, Other | N-acetylcysteine, Bromelain | Safety | Mild/Moderate Cases |
| ChiCTR2000 030259 |
Evaluation Danoprevir sodium tablets combined with ritonavir in the treatment of novel coronavirus infection (COVID-19): a randomized, open and controlled trial | China | Y | N | Drug Repurposing, Shelved drug or NME | Danoprevir, Ritonavir | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| DRKS000224 84 |
Clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2 (COVID-19) | Germany | N | N | Drug Repurposing | Hydrogen peroxide | Efficacy - Viral load | Mild/Moderate Cases |
| JPRN-jRCT2 071200023 |
Evaluation of Nelfinavir for asymptomatic and mild COVID-19: A multicenter open-label, blinded outcome, randomized controlled trial - NFV trial for asymptomatic and mild COVID-19 | Japan | Y | N | Drug Repurposing | Nelfinavir | Efficacy - Viral load | Mild/Moderate Cases |
| JPRN-jRCTs 051200036 |
Efficacy and safety of nintedanib on lung fibrosis in severe pneumonia induced by coronavirus disease 2019: Historical control study. | Japan | N | N | Drug Repurposing | Nintedanib | Efficacy - Mortality | Severe Cases |
| 2020-00203 9-31 |
COVIDSTORM - study protocol COVID-19: Slavage TOcilizumab as a Rescue Measure | Finland | N | N | Drug Repurposing | Tocilizumab | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| 2020-00203 8-33 |
Controlled clinical trial of hydroxychloroquine in the treatment of adult patients with Covid-19 infection in a primary care setting | Finland | Y | N | Drug Repurposing | Hydoxychloroquine | Efficacy - Other efficacy | Mild/Moderate Cases |
| 2020-00210 2-58 |
Mesenchymal stromal cell therapy for severe COVID-19 infection | Belgium | N | N | Cell/Blood based | ND:MSC | Safety | Severe Cases |
| 2020-00245 8-25 |
Randomized, controlled, open label, phase 2 clinical trial of Interferon-ß-1a (IFNß-1a) in COVID-19 patients. | Italy | Y | N | Drug Repurposing | Interferon beta 1a | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| 2020-00232 2-85 |
KAND567 Versus Placebo in Subjects Hospitalized with COVID-19. A Phase II, Randomized, 2-Arm Parallel-Group, Double-blind Study to Evaluate Efficacy, Safety, Tolerability, and Pharmacokinetics. | Sweden | Y | N | Shelved drug or NME | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases | |
| 2020-00148 3-28 |
A Randomized, Double-blind, Placebo-controlled Phase 1/2a Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of Ad26COVS1 in Adults Aged 18 to 55 Years Inclusive and Adults Aged 65 Years and Older | Belgium | Y | N | Shelved drug or NME | ND: Ad26COVS1 vaccine | Safety | General population |
| 2020-00257 4-27 |
A randomized, double-blind, placebo-controlled phase 2a and 2b study to evaluate the safety and efficacy of XAV-19 in patients with COVID-19 induced moderate pneumonia | France | Y | N | Shelved drug or NME | XAV-19 | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04510233 | Ivermectin Nasal Spray for COVID19 Patients | Egypt | Y | N | Drug Repurposing | Ivermectin | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04501796 | A Trial of NT-I7 in COVID-19 (SPESELPIS) | United States | Y | N | Shelved drug or NME | NT-17 | Safety | Mild/Moderate Cases |
| NCT04513158 | Convalescent Plasma in the Early Treatment of High-Risk Patients With SARS-CoV-2 (COVID-19) Infection | United States | N | N | Cell/Blood based | ND:Convalescent plasma | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04514302 | Safety and Efficacy of Anti-SARS-CoV-2 Equine Antibody Fragments (INOSARS) for Hospitalized Patients With COVID-19 | Mexico | Y | Y | Shelved drug or NME | INOSARS | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04504240 | Role of Famotidine in the Symptomatic Improvement of COVID-19 Patients | Bangladesh | Y | N | Drug Repurposing | Famotidine | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04500418 | Charité Trial of Cenicriviroc (CVC) Treatment for COVID-19 Patients | Germany | Y | N | Shelved drug or NME | Cenicriviroc | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04516746 | Phase III Double-blind, Placebo-controlled Study of AZD1222 for the Prevention of COVID-19 in Adults | United States | Y | N | Vaccine | AZD1222 | Efficacy - Infection rate (prevention) | General population |
| NCT04333732 | CROWN CORONATION: COVID-19 Research Outcomes Worldwide Network for CORONAvirus prevenTION | United States | Y | Y | Vaccine | MR Vaccine, MMRII Vaccine | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP), General population |
| NCT04497948 | Acalabrutinib Study With Best Supportive Care in Participants Hospitalized With COVID-19 | United States | N | N | Drug Repurposing | Acalabrutinib | Other | Severe Cases |
| NCT04513184 | Randomized Clinical Trial of Intranasal Dexamethasone as an Adjuvant in Patients With COVID-19 | Mexico | Y | N | Drug Repurposing | Dexamethasone | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04497298 | Clinical Trial to Evaluate the Safety and Immunogenicitiy of the COVID-19 Vaccine | France | Y | Y | Vaccine | TMV-083 Vaccine | Safety | General population |
| NCT04501978 | Therapeutics for Inpatients With COVID-19 | United States | Y | N | Drug Repurposing, Shelved drug or NME | LY3819253, Remdesivir | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04511819 | Losmapimod Safety and Efficacy in COVID-19 | United States | Y | N | Shelved drug or NME | Losmapimod | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04390594 | Efficacy and Safety Evaluation of Treatment Regimens in Adult COVID-19 Patients in Senegal | Senegal | Y | N | Drug Repurposing | Nafamostat | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04501783 | Study of Efficacy and Safety of TL-FVP-t vs. SOC in Patients With Mild to Moderate COVID-19 | Russian Federation | Y | N | Drug Repurposing | Favipiravir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases |
| NCT04498325 | Evaluating the Effect of NT-I7, a Long Acting Interleukin-7, to Increase Lymphocyte Counts and Enhance Immune Clearance of SARS-CoV-2 (COVID-19) | United States | Y | Y | Shelved drug or NME | NT-I7 | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04513470 | Allocetra-OTS in COVID-19 | Israel | N | N | Cell/Blood based | ND:Allocetra-OTS | Safety | Severe Cases |
| NCT04516954 | Convalescent Plasma for COVID-19 Patients | Viet Nam | N | N | Cell/Blood based | ND:Convalescent plasma | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04498273 | COVID-19 Positive Outpatient Thrombosis Prevention in Adults Aged 40-79 | United States | Y | Y | Drug Repurposing | Aspirin, Apixaban | Efficacy - Other efficacy | Mild/Moderate Cases, Other |
| NCT04504734 | Bucillamine in Treatment of Patients With COVID-19 | Canada | Y | N | Drug Repurposing | Bucillamine | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04497649 | Sofosbuvir Containing Regimens in Treatment of COVID 19 Patients | Egypt | Y | N | Drug Repurposing | Ledipasvir, Ribavirin, Daclatasvir, Sofosbuvir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04509999 | Bicalutamide to Block TMPRSS2 in Males With COVID-19 Infection | United States | Y | N | Drug Repurposing | Bicalutamide | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases, Other |
| NCT04505774 | Anti-thrombotics for Adults Hospitalized With COVID-19 | United States | Y | N | Drug Repurposing | Heparin | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04500132 | To Evaluate Safety and Efficacy of EC-18 in COVID-19 Infection to Pneumonia | Korea, Republic of | Y | N | Shelved drug or NME | EC-18 | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04498377 | Study of F-652 (IL-22:IgG2 Fusion Protein) in Patients With Moderate to Severe COVID-19 | China | Y | N | Shelved drug or NME | F-652 | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04502472 | Open-label Treatment of Severe Coronavirus Disease 2019 (COVID-19) With Convalescent Plasma | United States | N | N | Cell/Blood based | ND:Convelescent plasma | Efficacy - Clinical improvement (except WHO scale) | Severe Cases |
| NCT04501965 | Phytomedicines Versus Hydroxychloroquine as an Add on Therapy to Azythromycin in Asymptomatic Covid-19 Patients | Guinea | Y | N | Drug Repurposing, Other | Hydroxychloroquine, Azithromycine, ND:Phytomedicine | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04502342 | Add on to Azythromycine, Phytomedicine and/or Antimalarial Drug vs Hydroxychloroquine in Uncomplicated COVID-19 Patients | Guinea | Y | Y | Drug Repurposing, Other | Hydroxycloroquine, Azythromycine, Cospherunate, ND;Phytomedicine | Efficacy - Viral load | Mild/Moderate Cases |
| NCT04501952 | Study to Evaluate the Efficacy and Safety of Remdesivir (GS-5734™) Treatment of Coronavirus Disease 2019 (COVID-19) in an Outpatient Setting | United States | Y | N | Shelved drug or NME | Remdesivir | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04510207 | A Study to Evaluate The Efficacy, Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines (Vero Cell) in Healthy Population Aged 18 Years Old and Above | United Arab Emirates | Y | N | Vaccine | ND:Vaccine | Efficacy - Infection rate (prevention) | General population, Other |
| NCT04484493 | Corticosteroid Nasal Spray in COVID-19 Anosmia | Egypt | Y | N | Drug Repurposing | Mometasone furoate | Other | Other |
| NCT04497987 | A Study of LY3819253 (LY-CoV555) in Preventing SARS-CoV-2 Infection and COVID-19 in Nursing Home Residents and Staff | United States | Y | N | Shelved drug or NME | LY3819253 | Efficacy - Infection rate (prevention) | Exposed individuals (family members, HCP) |
| NCT04511650 | A Study to Evaluate the Safety and Efficacy of Razuprotafib, a Novel Tie 2 Activator, in Hospitalized Subjects With Moderate to Severe Coronavirus Disease 2019 (COVID-19) | United States | Y | N | Shelved drug or NME | Razuprotafib | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04512079 | FREEDOM COVID-19 Anticoagulation Strategy | United States | Y | N | Drug Repurposing | Apixaban | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04497389 | Safety and Feasibility of Amniotic Fluid as a Treatment for COVID-19 Patients | United States | Y | N | Cell/Blood based | ND:Amniotic Fluid | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04509973 | Higher vs. Lower Doses of Dexamethasone for COVID-19 and Severe Hypoxia | Denmark | Y | N | Drug Repurposing | Dexamethasone | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04499313 | Dexamethasone Vs Methylprednisolone for the Treatment of Patients With ARDS Caused by COVID-19 | Bangladesh | Y | N | Drug Repurposing | Dexamethasone, Methylprednisolone | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04517188 | Halodine Nasal Antiseptic in Patients With COVID-19 | United States | N | N | Shelved drug or NME | Povidone-Iodine | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04498247 | A Study to Assess Safety, Tolerability, and Immunogenicity of V591 (COVID-19 Vaccine) in Healthy Participants (V591-001) | United States | Y | N | Vaccine | ND:Vaccine | Safety | Other |
| NCT04500067 | Intravenous Immunoglobulin (IVIG, Bioven) Efficacy Assess for COVID-19 / SARS-CoV-2 Severe Pneumonia Complex Treatment | Ukraine | Y | N | Cell/Blood based | ND:Immunoglobulin | Efficacy - Clinical improvement (except WHO scale) | Mild/Moderate Cases, Severe Cases |
| NCT04508023 | A Study of Rivaroxaban to Reduce the Risk of Major Venous and Arterial Thrombotic Events, Hospitalization and Death in Medically Ill Outpatients With Acute, Symptomatic Coronavirus Disease 2019 (COVID-19) Infection | United States | Y | N | Drug Repurposing | Rivaroxaban | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
| NCT04510194 | MET-Covid Trial - METformin for Prevention and Outpatient Treatment of COVID-19 | United States | Y | N | Drug Repurposing | Metformin | Efficacy - Mortality | Mild/Moderate Cases, Severe Cases |
| NCT04510038 | Colchicine vs Current Standard of Care in Hospitalized Patients With COVID-19 and Cardiac Injury | United States | Y | N | Drug Repurposing | Colchicine | Efficacy - Mortality | Other |
| NCT04510402 | Phase I/II Trial of Povidone-iodine (PVP-I) Nasal Swab For Preventing COVID-19 Spread in Healthy Subjects | United Kingdom | N | Y | Shelved drug or NME | Povidone-Iodine | Safety | General population, Other |
| NCT04345419 | Remdesivir vs Chloroquine in Covid 19 | Egypt | Y | Y | Drug Repurposing | Chloroquine, Remdesivir | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04445220 | A Study of Cell Therapy in COVID-19 Subjects With Acute Kidney Injury Who Are Receiving Renal Replacement Therapy | United States | Y | N | Shelved drug or NME | SBI-101 | Safety | Other |
| NCT04498936 | Sofosbuvir/Ledipasvir and Nitazoxanide for Treatment of COVID-19 | Egypt | Y | N | Drug Repurposing | Ledipasvir/sofosbuvir, Nitazoxanide | Efficacy - Viral load | Mild/Moderate Cases, Severe Cases |
| NCT04517396 | Fenofibrate Intervention as COVID-19 Therapy | United States | Y | N | Drug Repurposing | Fenofibrate | Efficacy - WHO scale | Mild/Moderate Cases, Severe Cases |
| NCT04504032 | A Randomized, Controlled, Phase 2b Trial to Evaluate Safety and Efficacy of Rivaroxaban | United States | Y | N | Drug Repurposing | Rivaroxaban | Safety | Mild/Moderate Cases, Severe Cases |
| NCT04505592 | Tenecteplase in Patients With COVID-19 | United States | Y | N | Drug Repurposing | Tenecteplase | Efficacy - Oxygen parameters | Mild/Moderate Cases, Severe Cases |
| NCT04516941 | CorONa Virus edoxabaN ColchicinE (CONVINCE) COVID-19 | Switzerland | Y | Y | Drug Repurposing | Colchicine, Edoxaban | Efficacy - Other efficacy | Mild/Moderate Cases, Severe Cases |
